Summary
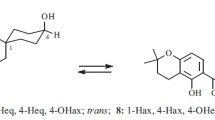
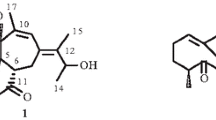
Two stereoisomers of “Quercus-Whisky” lactone (1) were isolated from oak wood. By means of spectroscopic data (GC/MS; FT/IR;1H-NMR) using authentical references these were identified as the cis/trans diastereoisomers of (1). Reductive cleavage by LiAIH4 produced stereoisomers of 3-methyloctan-1,4-diol, which were differentiated as their diastereomeric di-esters with (R)-Mosher acid and (S)-O-acetyllactic acid respectively. The optical purity of the naturally occurring stereoisomers was estimated by reference to optically pure compounds. The oak wood contained 77% of (3S,4S)-configuratedcis-1 and 23% of (3S,4R)-configuratedtrans-1.
Zusammenfassung
Durch Extraktion mit Diethylether lassen sich aus Eichenholz zwei Stereoisomere des „Quercus-/Whisky-Lactons” (1) isolieren, die mit Hilfe authentischer Vergleichssubstanzen über Spektrenvergleich (GC/MS; FT/IR;1H-NMR) als cis/trans Diastereomere von1 erkannt werden. Die reduktive Lactonspaltung voncis-1 bzw.trans-1 mit LiAlH4 liefert stereoisomere 3-Methyloctan-1,4-diole, die sich mit (R)-Moshersäurechlorid bzw. (S)-O-Acetylmilchsäurechlorid in trennbare diastereomere Di-Ester überführen lassen. Mit Hilfe optisch reiner Vergleichssubstanzen können die Stereoisomeren identifiziert und die optische Reinheit des natürlichen Whiskylactons beurteilt werden. Im Eichenholz werden 77% 3S,4S konfiguriertescis-1 und 23% 3S,4R konfiguriertestrans-1 gefunden.
Similar content being viewed by others
Literatur
Günther C, Mosandl A (1986) Liebigs Ann Chem 2112
Nykännen L, Nykännen J, Moring M (1984) In: Adda J (ed) Progress in flavour research. Elsevier, Amsterdam Oxford New York Tokyo, p 339
Günther C (1987) Dissertation, Universität Würzburg (in Vorbereitung)
Masuda M, Nishimura K (1971) Phytochemistry 10:1401
Laporte JF, Rambaud R (1966) C R Acad Sci Ser C 262:1095
Kepner RE, Webb AD, Müller CJ (1972) Am J Enol Vitic 23:103
Otsuka K, Zenibayashi Y, Itoh M, Totsuka A (1974) Agric Biol Chem 38:485
Pisarnitskii AF, Egorov IA, Gavrilov AJ (1976) Prikl Biokhim Mikrobiol 12:192
Litschew WJ (1976) Mitt Klosterneuburg 26:139
Guymon WJ, Crowell EA (1972) Am J Enol Vitic 23:114
Muller CJ, Kepner RE, Webb AD (1973) Am J Enol Vitic 24:5
Schreier P, Drawert F, Winkler F (1979) J Agric Food Chem 27:365
ter Heide R, de Valois PJ, Visser J, Jaegers PP, Timmer R (1978) Analysis of foods and beverages. Academic Press, New York, p 249
Jackman LM, Sternhell S (1969) Application of nuclear magnetic resonance spectroscopy in organic chemistry, chaps 3-8. Pergamon Press, Oxford
Masuda M, Nishimura K (1981) Chem Lett 1333
Moret E, Schlosser M (1984) Tetrahedron Lett 25:4491
Bardili B, Marschall-Weyerstahl H, Weyerstahl P (1985) Liebigs Ann Chem 1985:275
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Günther, C., Mosandl, A. Stereoisomere Aromastoffe. Z Lebensm Unters Forch 185, 1–4 (1987). https://doi.org/10.1007/BF01083330
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01083330