Summary
The mineral fibroferrite has the chemical formula Fe(OH)SO4·xH2O; the value forx has not been definitely settled, but as a rule it is found to be near five. Several symmetries are given in the literature.
A sample from Saint Felix de Paillères, France, proved to be rhombohedral with space group R3; lattice constants for the hexagonal cell area=24.176,c=7.656 Å. As calculated from the experimental density (ρ=1.95 g·cm−3)Z=18 for this cell. Intensities were collected on an automated X-ray diffractometer from a thin fiber extended along [00.1]. The structure was determined by Patterson and Fourier methods. Least squares refinement with 818 observed reflections resulted inR=0.076.
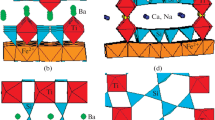
The structure contains hydroxo-bridged {Fe(OH)(H2O)2SO4} spiral chains built of [Fe(OH)2(H2O)2O2] octahedra and SO4 tetrahedra. Hydrogen bonds provide connections between these chains. The spiral chains are a stereoisomer variant of the hydroxo-bridged linear chains of Fe(OH)SO4, butlerite and parabutlerite. A comparison of these compounds is givenm to understand the relationship between the structure and their water content.
Zusammenfassung
Das Mineral Fibroferrit hat die chemische Formel Fe(OH)SO4·xH2O; der Wert furx scheint nicht endgültig geklärt zu sein, liegt aber meist nahe 5. Verschiedene Symmetrien werden in der Literatur angegeben.
Eine Probe von Saint Felix de Paillères, Frankreich, erwies sich als rhomboedrisch mit der Raumgruppe R3; die Gitterkonstanten der hexagonalen Zelle sinda=24,176,c=7,656 Å. Die experimentelle Bestimmung der Dichte (ρ=1,95 g·cm−3) führt für diese Zelle zuZ=18. Von einer nach [00.1] gestreckten dünnen Faser wurden die Intensitäten auf einem automatischen Röntgendiffraktometer gesammelt. Die Struktur wurde mit Patterson-und Fouriersynthesen gelöst. Eine Verfeinerung nach der Methode der kleinsten Quadrate führte für 818 beobachtete Reflexe aufR=0,076.
Die Struktur enthält durch Hydroxil-Gruppen verknüpfte {Fe(OH)(H2O)2SO4}-Spiralketten, die aus [Fe(OH)2(H2O)2O2]-Oktaedern und SO4-Tetraedern aufgebaut sind. Die Spiralketten von Fibroferrit sind eine stereoisomere Variante der annähernd linearen Fe−O−S-Ketten von Fe(OH)SO4, Butlerit und Parabutlerit. Diese Verbindungen werden mit Fibroferrit verglichen, um Beziehungen zwischen Struktur und Wassergehalt zu verstehen.
Similar content being viewed by others
References
Bandy, M. C., 1938: Mineralogy of three sulphate deposits of northern Chile. Amer. Min.23, 669–760.
Borène, J., 1970: Structure cristalline de la parabutlérite. Bull. Soc. fr. Min. Crist.93, 185–189.
Brown, I. D., Shannon, R. D., 1973: Empirical bond-strength-bond-length curves for oxides. Acta Crist.A 29, 266–282.
Busing, W. R., Martin, K. O., Levy, H. A., 1962: ORFLS report ORNLTM305, Oak Ridge National Laboratory, Oak Ridge Tennessee.
Césbron, F., 1964: Contribution à la minéralogie des sulfates de fer hydratés. Bull. Soc. fr. Min. Crist.87, 125–143.
Cromer, D. T., Waber, J. T., 1965: Scattering factors computed from relativistic Dirac-Slater wave functions. Acta Crist.18, 104–109.
Donnay, G., Donnay, J. D. H., 1973: Bond-valence summation for borates. Acta Crist.B29, 1417–1425.
Fanfani, L., Nunzi, A., Zanazzi, P. F., 1970: The crystal structure of roemerite. Amer. Min.55, 78–89.
———, 1971: The crystal structure of butlerite. Amer. Min.56, 751–757.
———Zanzari, A. R., 1973: The copiapite problem: the crystal structure of a ferrian copiapite. Amer. Min.58, 314–322.
Gordon, S. G., 1942: From Césbron. Bull. Soc. fr. Min. Crist.87, 125–143.
Hamilton, W. C., 1959: On the isotropic temperature factor equivalent to a given anisotropic temperature factor. Acta Crist.12, 609–610.
Johansson G., 1962: On the crystal structures of FeOHSO4 and InOHSO4. Acta Chem. Scand.16, 1234–1244.
Larsen, E. S., 1921: From Césbron. Bull. Soc. fr. Min. Crist.87, 125–143.
Moore, P. B., Araki, T., 1974a: Jahnsite, CaMn2+Mg2(H2O)8Fe 3+2 (OH)2[PO4]4: A novel stereoisomerism of ligands about octahedral corner-chains. Amer. Min.59, 964–973.
—— 1974b: Stewartite, Mn2+Fe 3+2 (OH)2(H2O)6[PO4]2·2H2O: its atomic arrangement. Amer. Min.59, 1272–1276.
Palache, C., Berman, H., Frondel, C. 1951: Dana's System of Mineralogy 7th ed., pp. 614–616. New York: Wiley.
Rossman, G. R., 1976: Spectroscopic and magnetic studies of ferric iron hydroxy sulfates: the series Fe(OH)SO4·nH2O and the Jarosite. Amer. Min.61, 398–404.
Scordari, F., 1978: The crystal structure of hohmannite, Fe2(H2O)4[(SO4)2O]·4H2O and its relationship to amarantite, Fe2(H2O)4 [(SO4)2O]·3 H2O. Min. Mag.42, 144–146 (SYNOPSIS). Full text in the miniprint section, M9-11.
Süsse, P., 1968: The crystal structure of amarantite. Z. Krist.127, 261–275.
Toussaint, J., 1956: From Césbron. Bull. Soc. fr. Min. Crist.87, 125–143.
Wan, C., Ghose, S., Rossman, G. R., 1978: Guldite, a layer structure with a ferric hydroxy-sulphate chain and its optical absorption spectra. Amer. Min.63, 478–483.
Author information
Authors and Affiliations
Additional information
With 2 Figures
Rights and permissions
About this article
Cite this article
Scordari, F. Fibroferrite: A mineral with a {Fe(OH) (H2O)2SO4} spiral chain and its relationship to Fe(OH)SO4, butlerite and parabutlerite. TMPM Tschermaks Petr. Mitt. 28, 17–29 (1981). https://doi.org/10.1007/BF01081848
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01081848