Summary
The crystal structure of wöhlerite (monoclinicP21,a=10.823(3,b=10.244(3),c=7.290(2) Å, β=109.00(4)o) was refined using 2343 independent diffractions toR 1=0.019 andR 2=0.025.
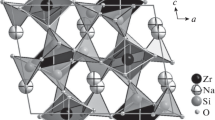
The refinement confirms the main features of the structure model proposed byShibayeva andBelov (1962), namely the walls of large cation polyhedra interconnected as in cuspidine, with Si2O7 groups linked to the polyhedra. At difference from the structure proposed by the quoted authors, both the Si2O7 groups are clinging to edges of calcium polyhedra.
The cation distribution in the polyhedral walls and the location of fluorine anions are clarified and discussed, resulting in the following crystal chemical formula Na4(Ca7.48Mn0.20Fe0.32)Zr2(Nb1.6Ti0.4) (Si2O7)4O4F2(O1.6F0.4).
Zusammenfassung
Die Kristallstruktur des Wöhlerits (monoklinP21,a 0=10,823(3),b 0=10,244(3),c 0=7,290(2) Å, β=109,00(4)o) wurde mit 2343 unabhängigen Reflexen aufR 1=0,019 undR 2=0,025 verfeinert.
Die Verfeinerung bestätigt im wesentlichen Züge des vonShibayeva undBelov (1962) vorgeschlagenen Strukturmodells, nämlich Bänder aus den Polyedern um die großen Kationen, die — wie im Cuspidin — durch an den Polyedern hängenden Si2O7-Gruppen verknüpft sind.
Die Kationenverteilung in den Polyederbändern und die Lokalisation der Fluor-Anionen wurden aufgeklärt und werden diskutiert; es ergibt sich folgende kristallchemische Formel Na4(Ca7,48 Mn0,20Fe0,32)(Zr2(Nb1,6 Ti0,4) (Si2O7)4O4F2(O1,6F0,4).
Similar content being viewed by others
References
Bayer, G., 1974: Crystalchemistry of Zirconium. In: Handbook of Geochemistry (Ed.K. H. Wedepohl).40A, 1–8, Berlin-Heidelberg-New York: Springer.
Belov, N. V., 1961: Crystal Chemistry of Large Cation Silicates. New York: Consultants Bureau.
Choisnet, J., D. Groult, B. Raveau, andM. Gasperin, 1977: Nouvelles structures à tunnels de section pentagonale K3Nb3B2O12 et K3Ta3B2O12. Acta Cryst.B33, 1841–1845.
Cleve, P. T., 1890: Zeit. Krist.16, 360.
Donnay, G., andR. Allmann, 1970: How to recognize O2−, OH− and H2O in crystal structures determined by X-rays. Amer. Min.55, 1003–1015.
Bibbs, G. V., M. M. Hamil, S. J. Louisnathan, L. S. Bartell, andHsiukang You, 1972: Correlation between Si−O bond length, Si−O−Si angle and bond overlap populations calculated using extended Huckel molecular orbital theory. Amer. Min.57, 1578–1613.
Hamilton, W. C., 1959: On the isotropic temperature factor equivalent to an anisotropic temperature factor. Acta Cryst.12, 609–610.
—, 1965: Significance tests on the crystallographicR factor. Acta Cryst.18, 502–510.
International Tables for X-ray Crystallography, Vol. IV, 1974, Ed.J. A. Ibers andW. C. Hamilton. Birmingham: Kynoch Press.
Li Te Yü, V. I. Simonov, andN. V. Belov, 1966: Crystal structure of niocalite. Dokl. Akad. Nauk. SSSR167, 566–569.
Megaw, H. D., 1968a: A simple theory of the off-centre displacement of cations in octahedral environments. Acta Cryst.B24, 149–153.
—, 1968b: The thermal expansion of interatomic bonds, illustrated by experimental evidence from certain niobates. Acta Cryst.A24, 589–604.
North, A. C. T., D. C. Phillips, andF. Scott Mathews, 1968: A semi-empirical method of absorption correction. Acta Cryst.A24, 351–359.
O'Keeffe, M., andB. G. Hyde, 1978: On Si−O−Si configurations in silicates. Acta Cryst.B 34, 27–32.
Saburi, S., A. Kawahara, C. Henmi, I. Kusachi, andK. Kihara, 1977: The refinement of the crystal structure of cuspidine. Min. Jour.8, 286–298.
Sakowski-Cowley, A. C., K. Lukaszewicz, andH. D. Megaw, 1969: The structure of sodium niobate at room temperature, and the problem of reliability in pseudosymmetric structures. Acta Cryst.B25, 851–865.
Shannon, R. D., andC. T. Prewitt, 1969: Effective ionic radii in oxides and fluorides. Acta Cryst.B25, 925–946.
Shibayeva, R. I., andN. V. Belov, 1962: Crystal structure of wöhlerite, Ca2Na(Zr, Nb) Si2O7(O, F)2. Dokl. Akad. Nauk. SSSR146, 897–900.
Simonov, V. I., andN. V. Belov, 1960: Crystal structure of lavenite. Dokl. Akad. Nauk. SSSR130, 167–170.
Smirnova, R. F., I. M. Rumanova, andN. V. Belov, 1955: Crystal structure of cuspidine. Zap. Vses. mineral. obshsch.87, 159.
Tschernik, G., 1909: Bull. Acad. Imp. Sc. St. Petersburg3, 903.
Author information
Authors and Affiliations
Additional information
With 3 Figures
Rights and permissions
About this article
Cite this article
Mellini, M., Merlino, S. Refinement of the crystal structure of wöhlerite. TMPM Tschermaks Petr. Mitt. 26, 109–123 (1979). https://doi.org/10.1007/BF01081296
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01081296