Abstract
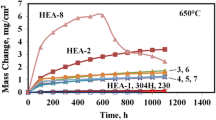
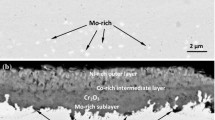
Four commercial alloys-Hastelloy C-4, alloy 1.4306S (SS 304L), Incoloy 800H, and Incoloy 825-were studied for their oxidation behavior at elevated temperatures. Specimens were exposed to air from 600 to 1200°C for 1 to 400 hr. Reaction kinetics of oxidation were determined, and the morphology of the surface-oxide scales was investigated. Hastelloy C-4 showed better resistance to oxidation dor exposure temperatures up to 1000°C in comparison with the other three alloys. In this temperature range, it follows a cubic rate law of oxidation due to formation of uniform, protective, and adherent oxide scales. The latter three alloys obeyed the parabolic rate law at 1000°C and 1200°C, but for lower temperatures a mixed behavior was shown. The oxide layer developed on the alloy 1.4306S was always in the form of stratified nodules/warts. For longer exposures the nodules joined each other to form continuous but discrete layers. Incoloy 800H and Incoloy 825 behaved in an almost identical manner, their reaction kinetics being governed by the parabolic rate law throughout the temperature range. Oxide spalling was observed at all temperatures. In contrast to Incoloy 800H the Incology 825 was totally oxidized for longer exposures at 1200°C.
Similar content being viewed by others
References
P. Kofstad, A. Rahmel, R. A. Rapp, and D. L. Douglass,Oxid. Met. 32, 125 (1989).
G. Rundell and J. McConnell,Oxid. Met. 36, 353 (1991).
N. Hussain, G. Schanz, S. Leistikow, and K. A. Shahid,Oxid. Met. 32, 405 (1989).
W. Z. Friend,Corrosion of Nickel and Nickel-Base Alloys (Wiley, New York, 1980), p. 349.
Manly, et al., Am. Soc. Testing Materials Standards, part 3, 173 (1964).
G. C. Wood,Oxid. Met. 2, 42 (1970).
A. Fursey, B. Kent, and S. R. T. Saunders,J. Microsc. 99, 147 (1973).
G. C. Wood,Corros. Sci. 2, 255 (1962).
G. C. Wood and D. P. Whittle,Corros. Sci. 2, 263 (1964).
J. T. Bittel, L. H. Sjodahl, and J. F. White,Corrosion 25, 7 (1969).
F. H. Stott, F. I. Wei, and C. A. Enahoro,Werkst. Korros. 40, 198 (1989).
A. L. Marasco and D. J. Young,Oxid. Met. 36, 157 (1991).
D. L. Douglass and F. Rizzo-Assuncao,Oxid. Met. 29, 272 (1988).
F. Gesmundo, D. de Asmundis, G. Battilana, and E. Ruedl,Werkst. Korros. 38, 368 (1987).
E. A. Polman, T. Fransen, and P. J. Gelling,Oxid. Met. 33, 135 (1990).
G. C. Wood,Corros. Sci. 2, 173 (1961).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hussain, N., Shahid, K.A., Khan, I.H. et al. Oxidation of high-temperature alloys (superalloys) at elevated temperatures in air: I. Oxid Met 41, 251–269 (1994). https://doi.org/10.1007/BF01080783
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01080783