Abstract
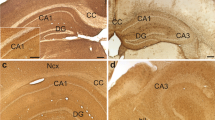
The presence of fatty acid-binding protein (FABP) in the embryonic chick retina may be linked to the demand for polyunsaturated fatty acids in this developing neural tissue. There is a decline in the overall level of FABP as the retina matures, suggesting a role for FABP in cellular differentiation. However, this pattern is not present in the chick brain, indicating a unique function for FABP in the retina. Immunohistochemical staining of paraffin sections of chick retina from embryonic day 21 revealed immunopositive photoreceptor inner segments, outer nuclear layer, ‘radial processes’ in the inner nuclear layer, a subpopulation of cells in the ganglion cell layer, and inner limiting membrane. This pattern suggested that FABP positive cells were photoreceptors, Müller (glial) cells, and possibly ganglion cells. Staining of sections for glutamine synthetase, an enzyme specific for Müller cells, was similar but not identical to the pattern observed with FABP; thus identification of these cells as FABP-positive was not conclusive. However, in retinal cells dissociated from day E14 embryos and cultured for one week, staining with FABP was more intense in the neurons than in the ‘flat’ cells (presumed to be derived from the Müller cells). Retinal FABP thus appears to be localized predominantly in neurons, and may serve to sequester fatty acids in preparation for neurite outgrowth as the retinal cells differentiate.
Similar content being viewed by others
Abbreviations
- FABP:
-
Fatty Acid-Binding Protein
- PUFA:
-
Polyunsaturated Fatty Acid
References
Fliesler SJ, Anderson RE: Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res 22: 79–131, 1983
Sellner PA, Clough JA: Fatty acid composition of phospholipids from chick neural retina during development. Exp Eye Res 54: 725–730, 1992
Bass NM: The cellular fatty acid binding proteins: aspects of structure, regulation, and function. Int Rev Cytol 111: 143–184, 1988
Matarese V, Stone RL, Waggoner DW, Bernlohr DA: Intracellular fatty acid trafficking and the role of cytosolic lipid binding proteins. Prog Lipid Res 28: 245–272, 1989
Paulussen RJA, Veerkamp JH: Intracellular fatty-acid-binding proteins: characteristics and function. In: HJ Hilderson (ed.) Subcellular Biochemistry, Vol. 16. Plenum Press, New York, 1990, pp 175–226
Paulussen RJA, Van Der Logt CPE, Veerkamp JH: Characterization and binding properties of fatty acid-binding proteins from human, pig and rat heart. Arch Biochem Biophys 264: 533–545, 1988
Lee L, Wiggert B: Isolation and characterization of an unsaturated fatty acid-binding protein from developing chick neural retina. J Neurochem 42: 47–53, 1984
Sellner PA, Phillips AR: Phospholipid synthesis by chick retinal microsomes: fatty acid preference and effect of fatty acid binding protein. Lipids 26: 62–67, 1991
Lowry OH, Rosebrough JN, Farr AL, Randall RJ: Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–275, 1951
Venuti SE, Helmkamp Jr GM: Tissue distribution, purification and characterization of rat phosphatidylinositol transfer protein. Biochim Biophys Acta 946: 119–128, 1988
Adler R: Preparation, enrichment, and growth of purified cultures of neurons and photoreceptors from chick embryos and from normal and mutant mice. In: Methods in Neuroscience, Vol. 2. Academic Press, 1990, pp 134–150
Ramón y Cajal S: The structure of the retina. Compiled and translated by SA Thorpe, M Glickstein. CC Thomas, Springfield, IL, 1972, plate VI and pp 172–3
Li H-P, Sheffield JB: Isolation and characterization of flat cells, a subpopulation of the embryonic chick retina. Tiss Cell 16: 843–857, 1984
Schoentgen F, Bonanno LM, Pignède G, Jollès P: Amino acid sequence and some ligand binding properties of fatty acid-binding protein from bovine brain. Mol Cell Biochem 98: 35–39, 1990
Futterman S, Saari JC: Metabolism and photochemistry in the retina. In: RA Moses (ed.) Adler's Physiology of the Eye. CV Mosby Co, St Louis, MO, 1981, pp 411–426
Moscona AA, Linser PJ: Developmental and experimental changes in retinal glia cells: cell interactions and control of phenotype expression and stability. Curr Top Devel Biol 18: 155–188, 1983
Moore SA, Yoder E, Murphy S, Dutton GR, Spector AA: Astrocytes, not neurons, produce docosahexaenoic acid (22:6ω-3) and arachidonic acid (20:4ω-6). J Neurochem 56: 518–524, 1991
Grün G: The development of the vertebrate retina: a comparative survey. Adv Anat Embr Cell Biol 78: 1–83, 1982
Prada C, Puga J, Pérez-Méndez L, López R, Ramírez G: Spatial and temporal patterns of neurogenesis in the chick retina. Eur J Neurosci 3: 559–569, 1991
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sellner, P.A. Retinal FABP principally localizes to neurons and not to glial cells. Mol Cell Biochem 123, 121–127 (1993). https://doi.org/10.1007/BF01076483
Issue Date:
DOI: https://doi.org/10.1007/BF01076483