Abstract
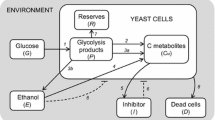
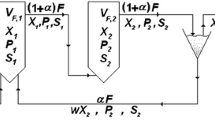
Substrate concentration gradients are likely to appear during large scale fermentations. To study effects of such gradients on microorganisms, an aerated scale-down reactor system was constructed. It consists of a plug flow reactor (PFR) and a stirred tank reactor (STR), between which the medium is circulated. The PFR, which is an aerated static mixer reactor, was characterized with respect to plug flow behaviour and oxygen transfer. A Bodenstein number of 15–220, depending on residence time and aeration rate, and a kLa of 500–1130 h−1, depending mainly on aeration rate, were obtained. The biological test system used, was aerobic ethanol production by Saccharomyces cerevisiae, due to sugar excess. The ethanol concentration profile and the yield of biomass were compared in two fed-batch fermentations. In the first case, the feeding point of molasses was located at the inlet of the PFR. This simulates location of the feeding point in the segregated part of a heterogeneous reactor, with local high sugar concentrations. In the second mode of operation, as a control with good mixing conditions, the PFR was disconnected from the STR, into which the substrate was fed. Differences were found: Up to 6% less biomass was produced and a larger amount of ethanol was formed in the two-compartment reactor system, due to the uneven sugar concentration distribution. This emphasizes the importance of the location of, and the mixing conditions at, the feeding point in a bioreactor.
Similar content being viewed by others
References
Oosterhuis, N. M. G.; Kossen, N. W. F.: Dissolved oxygen concentration profiles in a production-scale bioreactor. Biotechnol. Bioeng. 26 (1984) 546–550
Larsson, G.; Enfors, S.-O.: Influence of oxygen starvation on the respiratory capacity of Penicillium chrysogenum. Appl. Microbiol. Biotechnol. 21 (1985) 228–233
Larsson, G.; Enfors, S.-O.: Studies of insufficient mixing in bioreactors: Effects of limiting oxygen concentrations and short term oxygen starvation on Penicillium chrysogenum. Bioproc. Eng. 3 (1988) 123–127
Larsson, G.: Use of microbial metabolism to predict and control the bioreactor performance. Ph.D. thesis (ISBN 91-7170-099-9), KTH, Stockholm, Sweden, 1990
Sweere, A. P. J.; Matla, Y. A.; Zandvliet, J.; Luyben, K. Ch. A. M.; Kossen, N. W. F.: The influence of continually changing glucose concentrations on the fed-batch Baker's yeast production. Appl. Microbiol. Biotechnol. 28 (1988) 109–115
van Urk, H.; Mak, P. R.; Scheffers, W. A.; van Dijken, J. P.: Metabolic responses of Saccharomyces cerevisiae CBS 8066 and Candida utilis CBS 621 upon transition from glucose limitation to glucose excess. Yeast 4 (1988) 283–291
Sonnleitner, B.; Käppeli, O.: Growth of Saccharomyces cerevisiae is controlled by its limited respiratory capacity: Formulation and verification of a hypothesis. Biotechnol. Bioeng. 28 (1986) 927–937
Enfors, S.-O.; Hedenberg, J.; Olsson, K.: Simulation of the dynamics in the Baker's yeast process. Bioproc. Eng. 5 (1990) 191–198
Schumpe, A.; Adler, I.; Deckwer, W.-D.: Solubility of oxygen in electrolyte solutions. Biotechnol. Bioeng. 20 (1978) 145–150
Schumpe, A.; Deckwer, W.-D.: Estimation of O2 and CO2 solubilities in fermentation media. Biotechnol. Bioeng. 21 (1979) 1075–1078
Levenspiel, O.: Chemical Reaction Engineering, 2nd edition, pp. 253–281. New York: John Wiley and Sons Inc. 1972
Moser, A.: Tubular fermenter for aerobic processes. I: Physical characteristics. Biotech. Bioeng. Symposium 4 (1973) 399–411
Sinclair, G.: Formulation of the equations for oxygen transfer in fermenters. Biotechnol. Lett. 6 (1984) 65–70
Robinson, C. W.; Wilkie, C. R.: Oxygen absorption in stirred tanks: A correlation for ionic strength effects, Biotechnol. Bioeng. 15 (1973) 755–782
Ruchti, G.; Dunn, I. J.; Bourne, J. R.; von Stockar, U.: Practical guidelines for the determination of oxygen transfer coefficients (KLa) with the sulphite oxidation method. Chem. Eng. J. 30 (1985) 29–38
Linek, V.; Vacek, V.: Chemical engineering use of catalyzed sulfite oxidation kinetics for the determination of mass transfer characteristics of gas-liquid contractors. Chem. Eng. Sci. 36 (1981) 1747–1768
Fowler, J. D.; Dunlop, E. H.: Effects of reactant heterogeneity and mixing on catabolite repression in cultures of Saccharomyces cerevisiae. Biotechnol. Bioeng. 33 (1989) 1039–1046
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
George, S., Larsson, G. & Enfors, S.O. A scale-down two-compartment reactor with controlled substrate oscillations: Metabolic response of Saccharomyces cerevisiae. Bioprocess Engineering 9, 249–257 (1993). https://doi.org/10.1007/BF01061530
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01061530