Abstract
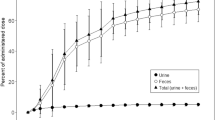
This study was conducted to evaluate the influence of route of administration upon the bioavailability and pharmacokinetics of methylprednisolone sodium succinate. Fourteen healthy adult male volunteers received 40 mg doses of methylprednisolone as the following treatments after an overnight fast in a 4-way crossover design: (a) as a 1 ml i.v. bolus;(b) as a 1 ml i.m. injection;(c) administered as an oral solution;and (d) as 5×8 mg oral tablets. Both the ester and free methylprednisolone were determined in plasma and urine. Study results indicate that the ester is rapidly and extensively converted to free methylprednisolone after all routes. The extent of methylprednisolone absorption was equivalent after i.v. and i.m. administration. Both orally administered treatments resulted in a lower extent of absorption attributed to a first-pass effect. Although a slightly lower extent of absorption was demonstrated following the oral administration of the methylprednisolone sodium succinate solution relative to the methylprednisolone oral tablets, this average difference of 9% would probably be of minimal therapeutic importance.
Similar content being viewed by others
References
B. D. Anderson and V. Taphouse. Initial rate studies of hydrolysis and acyl migration in methylprednisolone 21-hemisuccinate and 17-hemisuccinate.J. Pharm. Sci. 70:181–186 (1981).
D. C. Garg, J. W. Wagner, E. Sakmar, D. J. Weidler, and K. S. Albert. Rectal and oral absorption of methylprednisolone acetate.Clin. Pharmacol. Ther. 26:232–239 (1979).
K. S. Albert, S. W. Brown, Jr., K. A. DeSante, A. R. DiSanto, R. D. Stewart, and T. T. Chen. Double latin square study to determine variability and relative bioavailability of methylprednisolone.J. Pharm. Sci. 68:1312–1316 (1979).
P. K. Narang, R. Wilder, D. C. Chatterji, R. L. Yeager, and J. F. Gallelli. Systemic bioavailability and pharmacokinetics of methylprednisolone in patients with rheumatoid arthritis following “high-dose” pulse administration.Biopharm. Drug Dispos. 4:233–248 (1983).
W. A. Colburn and R. H. Butler. Radioimmunoassay for methylprednisolone.Steroids 22:687–698 (1978).
A. Rescigno and G. Segre. Turnover. InDrug and Tracer Kinetics. Blaisdell, Waltham, Mass., 1966, p. 161.
J. C. K. Loo and S. Riegelman. New method for calculating the intrinsic absorption rate of drugs.J. Pharm. Sci. 57:918–928 (1968).
C. M. Metzler, G. L. Elfring, and A. J. McEwen. A package of computer programs for pharmacokinetic modeling.Biometrics 30:562 (1974).
A. J. Sedman and J. G. Wagner. C-Strip, a Fortran IV computer program for obtaining initial polyexponential parameter estimates.J. Pharm. Sci. 65:1006–1010 (1976).
J. G. Wagner. Linear compartmental models. InFundamentals of Clinical Pharmacokinetics. Drug. Intelligence Publ., Hamilton, Ill., 1975, p. 102.
M. Rowland. Influence of route of administration on drug availability.J. Pharm. Sci. 67:70–74 (1972).
R. A. Waller and D. B. Duncan. A Bayes rule for the symmetric multiple comparison problem.J. Am. Stat. Assoc. 64:1484–1499 (1969).
K. S. Albert, A. J. Sedman, and J. G. Wagner. Pharmacokinetics of orally administered acetaminophen in man.J. Pharmacokin. Biopharm. 2:321–393 (1974).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Antal, E.J., Wright, C.E., Gillespie, W.R. et al. Influence of route of administration on the pharmacokinetics of methylprednisolone. Journal of Pharmacokinetics and Biopharmaceutics 11, 561–576 (1983). https://doi.org/10.1007/BF01059057
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01059057