Abstract
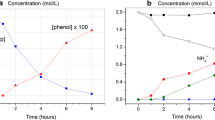
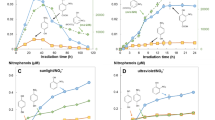
The photochemical formation of hydrogen peroxide in tryptophan solutions and natural waters was monitored by a sensitive spectrophotometric assay. Peroxide was characterized by ion exchange properties and mobility via thin-layer chromatography. Tryptophan photodecomposed in sunlight or near ultraviolet light (λ > 283 nm) with the formation of uncharacterized, acidic photoproducts and hydrogen peroxide; other oxidants (i.e. organic peroxides) were not detected in tryptophan photolysis mixtures. After brief exposure to sunlight, hydrogen peroxide in natural waters varied from below the detection limit (1.5μM) to 6.8μM; in highly eutrophic samples peroxide was formed in excess of 30μM. A humic acid soil extract, tyrosine, and several aromatic compounds including substituted phenols and anilines also generated stable photochemical oxidants suggesting that many compounds may serve as sources for hydrogen peroxide in natural waters.
Similar content being viewed by others
References
Asquith, R. S., and D. E. Rivett: Studies on the photooxidation of tryptophan. Biochim. Biophys. Acta252, 111 (1971).
Beer, R. J. S., T. Donavanik, and A. Robertson: Peroxides of tetrahydrocarbazole and related compounds. Part VI. Some indoleninyl hydroperoxides. J. Chem. Soc. 4139 (1954).
Bent, D. V., and E. Hayon: Excited state chemistry of aromatic amino acids and related peptides. III. Tryptophan. J. Am. Chem. Soc.97, 2612 (1975).
Bonneau, R., R. Pottier, O. Bagno, and J. Joussot-Dubien: pH dependence of singlet oxygen production in aqueous solutions using thiazine dyes as photosensitizers. Photochem. Photobiol.21, 159 (1975).
Crosby, D. G.: Experimental approaches to pesticide photo-decomposition. Residue Reviews25, 1 (1969).
Draper, W. M., and D. G. Crosby: Hydrogen peroxide and hydroxyl radical: Intermediates in indirect photolysis reactions in water. J. Agric. Food Chem.29, 699 (1981a).
—: Sensitive, enzyme-catalyzed, chromogenic reagent for hydrogen peroxide and other peroxygen compounds on TLC plates. J. Chromatogr.216, 413 (1981b).
Draper, W. M., D. G. Crosby, and J. B. Bowers: Measurement of photochemical oxidants in agricultural field water. Proceedings of the 172nd National Meeting of the American Chemical Society, San Francisco, CA (1976).
Gjessing, E. T.: Humic substances in natural water: Methods for separation and characterization, In H. L. Golterman and R. S. Clymo (eds.): Chemical environment in the aquatic habitat. Amsterdam: Noord-Hollandsch Uitgevers Maatschappij (1967).
Grossweiner, L. I., and H. Joschek: Optical generation of hydrated electrons from aromatic compounds, In R. F. Gould (ed.): Solvated electron. Washington, DC: American Chemical Society (1965).
Hart, E. J., and M. Anbar: The hydrated electron. New York: Wiley-Interscience (1970).
Larson, R. A., K. Smykowski, and L. L. Hunt: Occurrence and determination of organic oxidants in rivers and wastewaters. Chemosphere10, 1335 (1981).
McCormick, J. P., J. R. Fischer, J. P. Pachlatko, and A. Eisenstark: Characterization of a cell-lethal product from the photooxidation of tryptophan; hydrogen peroxide. Science191, 468 (1976).
McCormick, J. P., and T. Thomason: Near-ultraviolet photooxidation of tryptophan. Proof of formation of superoxide ion. J. Am. Chem. Soc.100, 312 (1978).
Mill, T., D. G. Hendry, and H. Richardson: Free-radical oxidants in natural waters. Science207, 886 (1980).
Mottola, H., B. Simpson, and G. Gorin: Absorptiometric determination of hydrogen peroxide in submicrogram amounts with leuco crystal violet and peroxidase as catalyst. Anal. Chem.42, 410 (1970).
Richardson, A., and E. Fortey: Action of light on amyl alcohol. J. Chem. Soc.69, 1349 (1896).
Ross, R., and D. G. Crosby: Characterization of photosensitizers in agricultural water. Proceedings of the 167th National Meeting of the American Chemical Society, Philadelphia, PA (1975).
Schnitzer, M., and S. U. Khan: Humic substances in the environment. New York: Marcel Dekker (1972).
Sinel'nikov, V. E.: Hydrogen peroxide level in river water, and methods for detecting it. Gidrobiol. Zh.7, 1 (1971a).
—: Experimental study of separate stages in the mechanism of oxidation of organic matter in waters by a chemiluminescent method. Akad. Nauk.20, 159 (1971b).
Soderquist, C. J., J. B. Bowers, and D. G. Crosby: The dissipation of molinate in a rice field. J. Agric. Food. Chem.25, 940 (1977).
Van Baalen, C. and J. E. Marler: Occurrence of hydrogen peroxide in sea water. Nature211, 951 (1966).
Witkop, B., and J. B. Patrick: On the mechanism of oxidation. 1. A new type of peroxide rearrangement catalyzed by acid. J. Am. Chem. Soc.73, 713 (1951).
Zepp, R. G., N. L. Wolfe, G. L. Baughman, and R. C. Hollis: Singlet oxygen in natural waters. Nature207, 421 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Draper, W.M., Crosby, D.G. The photochemical generation of hydrogen peroxide in natural waters. Arch. Environ. Contam. Toxicol. 12, 121–126 (1983). https://doi.org/10.1007/BF01055010
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01055010