Abstract
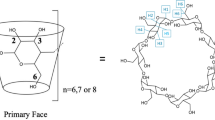
The observation that per-2,6-O-methyl-3-O-benzoyl-α-cyclodextrin (1) displays some unusual conformational behaviour in solution has led to a detailed investigation by (dynamic) NMR spectroscopy of the equilibration process that occurs in solutions of per-2,3-O-benzoyl-α-cyclodextrin (3) and some related compounds (7–9) between conformational isomers with averagedC 6 andC 3 molecular symmetries in certain organic solvents such as benzene, dichloromethane, and chloroform. The solvent dependence of the conformational equilibrium is also reflected in a spread of values for the specific optical rotations for 3 from +9° in 1,1,2,2-tetrachloroethane, where there is a degenerate equilibrium between species withC 3 molecular symmetry, to +92° in acetone where a species with averagedC 6 symmetry is present.
Similar content being viewed by others
References
J. F. Stoddart: ‘A Century of Cyclodextrins’,Carbohydr. Res. 192, xii, (1989).
A. Villiers:C. R. Acad. Sci. 112, 536 (1891).
W. Saenger: inInclusion Compounds, Vol. 2, eds. J. L. Atwood, J. E. D. Davies, and D. D. MacNicol, Academic Press, London, p. 231 (1984).
J. Szejtli:Cyclodextrins and their Inclusion Complexes, Akadémiai Kiado, Budapest (1982); D. Duchêne (Ed.),Cyclodextrins and Their Industrial Uses, Editions de Santé, Paris, (1987); J. Szejtli,Cyclodextrin Technology, Kluwer Academic Publishers, Dordrecht (1988); M. L. Bender and M. Komiyama, Cyclodextrin Chemistry, Springer, Berlin (1978).
A. P. Croft and R. A. Bartsch:Tetrahedron 39, 1417 (1983).
I. Tabushi: inInclusion Compounds, Vol. 3, eds. J. L. Atwood, J. E. D. Davies, and D. D. MacNicol, Academic Press, London, p. 445 (1984). R. Breslow inInclusion Compounds, Vol. 3, eds. J. L. Atwood, J. E. D. Davies, and D. D. MacNicol, Academic Press, London, p. 473 (1984).
R. Bergeron: inInclusion Compounds, Vol. 2, eds. J. L. Atwood, J. E. D. Davies, and D. D. MacNicol, Academic Press, London, p. 391 (1984).
K. Takeo, K. Uemura, and H. Mitch:J. Carbohydr. Chem. 7, 293 (1988); K. Takeo, H. Mitch, and K. Uemura:Carbohydr. Res. 187, 203 (1989).
K. Harata, K. Uekama, M. Otagiri, and F. Hirayama:Bull. Chem. Soc. Jpn. 60, 497 (1987); K. Harata:J. Chem. Soc. Chem. Commun. 928 (1988); K. Harata, K. Uekama, T. Imai, F. Hirayama, and M. Otagiri:J. Incl. Phenom. 6, 443 (1988).
D. R. Alston, P. R. Ashton, T. H. Lilley, J. F. Stoddart, R. Zarzycki, A. M. Z. Slawin, and D. J. Williams:Carbohydr. Res. 192, 259 (1989).
C. M. Spencer, J. F. Stoddart, and R. Zarzycki:J. Chem. Soc. Perkin Trans 2 1323 (1987).
J. Boger, R. J. Concoran, and J.-M. Lehn:Helv. Chim. Acta 61, 2190 (1978).
M. Karplus:J. Chem. Phys. 30, 11 (1959).
J. A. Schwarz and A. S. Perlin:Can. J. Chem. 50, 3667 (1972); A. Parfondry, N. Cyr, and A. S. Perlin:Carbohydr. Res. 59, 299 (1977).
B. Casu, M. Reggiani, G. G. Gallo, and A. Vigevani:Tetrahedron 22, 3061 (1966).
M. St Jacques, P. R. Sundarajan, K. J. Taylor, and R. H. Marchessault:J. Am. Chem. Soc. 98, 4386 (1976).
I. O. Sutherland:Ann. Rep. NMR Spectrosc. 4, 41 (1971).
J. F. Stoddart:The Stereochemistry of Carbohydrates, Wiley Interscience (1971).
R. U. Lemieux, T. L. Nagabkushan, and B. Paul:Can. J. Chem. 50, 773 (1970).
G. K. Hamer, F. Balza, N. Cyr, and A. S. Perlin:Can. J. Chem. 56, 3109 (1978).
A. Bax and R. Freeman:J. Am. Chem. Soc. 104, 1099 (1982).
B. Mulloy, T. A. Frienkiel, and D. B. Davies:Carbohydr. Res. 184, 39 (1988).
B. Klar, B. Hingerty, and W. Saenger:Acta Crystallogr. B36, 1154 (1980).
For example; P. Dais, T. K. M. Shing, and A. S. Perlin:J. Am. Chem. Soc. 106, 3082 (1984); P. Dais and A. S. Perlin:Magn. Res. Chem. 26, 373 (1988); G. M. Lipkind, A. S. Shashkov, S. S. Mamgan, and N. K. Kochetkov:Carbohydr. Res. 181, 1 (1988).
R. P. Veregin, C. A. Fyfe, R. H. Marchessault, and M. G. Taylor:Carbohydr. Res. 160, 41 (1987); H. Saito, G. Izumi, T. Mamizuka, S. Suzuki, and R. Tabeta:J. Chem. Soc. Chem. Commun. 1386 (1982).
K. Bock, A. Brignole, and B. W. Sigurskjold:J. Chem. Soc. Perkin Trans. 2 1711 (1986); A. S. Shashkov, G. M. Lipkind, Y. A. Knirel, and N. K. Kochetkov:Magn. Res. Chem. 26, 735 (1988).
J. Ronayne and D. H. Williams:Ann. Rep. NMR Spectrosc. 2, 83 (1969).
Y. Yamamoto, M. Onda, Y. Takahishi, Y. Inoue, and R. Chûjô:Carbohydr. Res. 170, 229 (1987).
D. Gutsche:The Calixarenes, Monographs in Supramolecular Chemistry, Series Ed. J. F. Stoddart, RSC, London, Ch. 4 (1990).
D. A. Rees:J. Chem. Soc. (B) 877 (1970); D. A. Rees and D. Thom:J. Chem. Soc. Perkin Trans. 2 191 (1977).
E. M. Kosower: inIntroduction to Physical Organic Chemistry, Wiley, New York, (1968); C. Reichardt:Angew. Chem. Int. Ed. Engl. 4, 29 (1965); E. L. Eliel and O. Hofer:J. Am. Chem. Soc. 95, 8041 (1973).
D. J. Williams: unpublished observations.
Author information
Authors and Affiliations
Additional information
This paper is dedicated to the memory of the late Dr C. J. Pedersen.
Rights and permissions
About this article
Cite this article
Ellwood, P., Spencer, C.M., Spencer, N. et al. Conformational mobility in chemically-modified cyclodextrins. J Incl Phenom Macrocycl Chem 12, 121–150 (1992). https://doi.org/10.1007/BF01053857
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01053857