Abstract
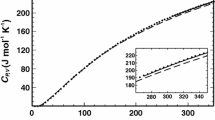
The free energy, entropy and enthalpy of sodium feldspar in thermal equilibrium and in metastable states are derived from investigations of the heat capacities of albite, analbite, ordered and disordered Or31. The lattice strains of all stable and metastable states are calculated from the two-order parameter theory published in the preceding paper. This approach also allows one to distinguish between the influence of Al, Si order and of the displacive lattice distortion on the thermodynamic behaviour of albite. The thermal cross-over between high albite and low albite is found to be a smooth function of temperature.
Similar content being viewed by others
References
Bruce AD, Cowley RA (1981) Structural Phase Transitions. Taylor and Francis, London
Carpenter MA, McConnell JDC, Navrotsky A (1984) Enthalpies of ordering in the plagioclase feldspar solid solution. To be published
Ditmars DA, Douglas TB (1971) Measurement of the relative enthalpy of pure α-Al2O3 (NBS heat capacity and enthalpy standard reference material No. 720) from 273 to 1,173 K. J Res 75 A No. 5: 401–420
Goldsmith JR, Jenkins DM (1984) The high-low albite relations revealed by hydrothermal melting and reversal of degree of order at high pressures. To be published
Grundy HD, Brown WL (1969) A high-temperature X-ray study of the equilibrium forms of albite. Mineral Mag 37 No 286: 156–172
Harlow GE, Brown GE, Hamilton WC (1973) Neutron diffraction study of Amelia low albite. Trans Am Geophys Union 54: 497 (abstr.)
Holm JL, Kleppa OJ (1968) Thermodynamics of the disordering process in albite. Am Mineral 53: 123–133
Kroll H, Bambauer HU, Schirmer U (1980) The high albite-monalbite and analbite-monalbite transitions. Am Mineral 65: 1192–1211
Mc Connell JDC, McKie D (1960) The kinetics of the ordering process in triclinic NaAl Si3O8. Mineral Mag 32: 436–454
O'Neill MJ (1966) Measurement of specific heat functions by differential scanning calorimetry. Anal Chem 38: 1331–1336
Salje E (1985) Thermodynamics of sodium feldspar I: order parameter treatment and strain induced coupling effects. Phys Chem Minerals 12: 93–98
Salje E, Devarajan V (1985) Phase transitions in systems with strain-induced coupling between two order parameters. Phase Transitions: submitted
Salje E, Wruck B (1983) Specific-heat measurements and critical exponents of the ferroelastic phase transition in Pb3(PO4)2 and Pb3(P1−xAsxO4)2. Phys Rev B 28: 6510–6518
Senderov EE (1980) On the theory of Al, Si ordering in albite. Phys Chem Minerals 6: 251–268
Strob WD (1983) Strukturverfeinerung eines Tief-Mikroklins, Zusammenhänge zwischen 〈T — O〉-Abständen und Al, Si-Ordnungsgrad und metrische Variation in einer Tief-Albit/Tief-Mikroklin Mischkristallreihe. Inaugural Dissertation, Münster
Waldbaum DR (1968) Enthalpies of fusion of KAISi3O8 and NaAlSi3O8. J Amer Ceram Soc 51: 61–62
Wenk HR, Kroll H (1984) Analysis of\(P\bar 1, I\bar 1 and C\bar 1\) plagioclase structures. Bull Mineral 107: 467–487
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Salje, E., Kuscholke, B., Wruck, B. et al. Thermodynamics of sodium feldspar II: Experimental results and numerical calculations. Phys Chem Minerals 12, 99–107 (1985). https://doi.org/10.1007/BF01046834
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01046834