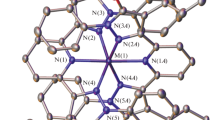
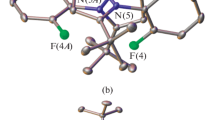
Summary
Iron(III) complexes of a quadridentate N2S2 donor ligand, 1,2-di(o-aminophenylthio)ethane (DAPTE) and its Schiff Base with salicylaldehyde, a hexadentate N2S2O2 donor ligand,viz. 1,2-di(o-salicylaldiminophenylthio)ethane (H2DSALPTE) have been synthesised and characterised.
The Schiff base ligand (1 mol) gave a dark green tri-iron(III) [Fe3(DSALPTE)(HDSALPTE)Cl3]Cl2 complex when reacted with anhydrous iron(III) chloride (1 mol). The Mössbauer data of this complex suggest the presence of three iron sites, one of which is octahedral and the other two tetrahedral. On the other hand, Fe(ClO4)3 reacted smoothly with H2DSALPTE in ethanol to give a mononuclear pseudo-octahedral complex in which the ligand functions in a dibasic hexadentate fashion. Mössbauer data suggest the presence of a low-spin-high-spin equilibrium in the solid state. The manganese(III) and cobalt(III) complexes of the Schiff base, H2DSALPTE, are also studied for the sake of comparison with the corresponding iron(III) complex. The N2S2 ligand, however, formed a low-spin pseudo-octahedral iron(III) complex. The complexes have been characterised by elemental analysis, molar conductance values, cryomagnetic data and i.r., electronic and Mössbauer spectral data.
Similar content being viewed by others
References
R. F. Gould (Ed.),Bioinorganic Chemistry, ACS Monograph No. 100, Amer. Chem. Soc, Washington D. C., (1971).
J. A. Bertrand, J. L. Breece, A. R. Kalyanaraman, G. J. Long and W. A. Baker Jr.,J. Am. Chem. Soc.,92, 5233 (1970).
J. A. Bertrand, J. L. Breece and P. G. Eller,Inorg. Chem.,13, 125 (1974) and ref. cited therein.
L. Cambi and L. Szego,Ber. Dtsch. Chem. Ges.,64, 2591 (1931).
M. M. Maltempo, T. H. Moss and M. A. Cusanouich,Biochim. Biophys. Acta,342, 290 (1974).
G. Mukherjee, S. N. Poddar and K. Dey,Ind. J. Chem.,25A, 275 (1986).
F. P. Dwyer, N. S. Gill, E. C. Gyarfas and F. Lions,J. Am. Chem. Soc.,76, 383 (1954).
R. D. Cannon, B. Chiswell and L. M. Venanzi,J. Chem. Soc. A., 1278 (1967).
C. A. McAuliffe, F. P. McCollough and A. Werfalli,Inorg. Chim. Acta,29, 57 (1978).
W. Levason, C. A. McAuliffe, F. P. McCullough and A. M. Werfalli,Inorg. Chim. Acta,25, 247 (1977).
H. Lux in G. Brauer (Ed.)Handbook of Preparative Inorganic Chemistry, Academic Press, 1965, 1469.
K. Nakamoto,Infrared and Raman Spectra of Inorganic and Coordination Compounds, Wiley, New York, 1978.
M. R. Rosenthall,J. Chem. Educ.,50, 331 (1973).
J. Catterick, P. Thornton and B. W. Fitzsimmons,J. Chem. Soc. Dalton Trans., 1420 (1977).
A. Vander Bergen, K. S. Murray, B. O. West and A. N. Buckley,J. Chem. Soc. A., 2051 (1969).
J. Mary Elizabathe and P. S. Zacharias,Polyhedron,6, 964 (1987).
G. R. Hall and D. N. Hendrickson,Inorg. Chem.,12, 2269 (1973).
K. R. Kunze, D. L. Perry and L. J. Wilson,Inorg. Chem.,16, 594 (1977).
H. Nakajima, T. Tanaka, H. Kobayashi and I. Tsujikawa,Inorg. Nucl. Chem. Lett.,12, 689 (1976).
M. Cox, J. Darken, B. W. Fitzsimmons, A. W. Smith, L. F. Larkworthy and K. A. Rogers,J. Chem. Soc. Dalton Trans., 1192 (1972).
B. F. Hoskin and C. D. Panna,Inorg. Nucl. Chem. Lett.,11, 409 (1975).
E. V. Dose, K. M. M. Murphy and L. J. Wilson,Inorg. Chem.,15, 2622 (1976).
M. F. Tweedle and L. J. Wilson,J. Am. Chem. Soc.,98, 4824 (1976).
R. H. Petty, E. V. Dose, M. F. Tweedle and L. J. Wilson,Inorg. Chem.,17, 1064 (1978).
W. D. Federer and D. N. Hendrickson,Inorg. Chem.,23, 3861 (1984).
M. D. Timken, D. N. Hendrickson and E. Sinn,Inorg. Chem.,24, 3947 (1985).
C. K. Jorgensen,Adv. Chem. Phys.,8, 47 (1965) and ref. cited therein.
E. Von Meerwal,Comp. Phys. Comm.,9, 117 (1975).
P. R. Edwards and C. E. Johnson,J. Chem. Phys.,49, 211 (1968).
N. N. Greenwood and T. C. Gibb,Mössbauer Spectroscopy, Chapman and Hall, London, p. 91, 1971.
Y. Maeda, N. Tsutsumi and Y. Yakashima,Inorg. Chem.,23, 2440 (1984).
A. B. P. Lever,Inorganic Electronic Spectroscopy, Elsevier, Amsterdam, 1968.
L. D. Jones and W. A. Runciman,Proc. Phys. Soc.,76, 996 (1960).
D. Attanasio, G. Dessy and V. Fares,Inorg. Chim. Acta,104, 105 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mukherjee, G., Poddar, S.N., Choudhury, K. et al. NS and NSO-Donor ligands and their metal complexes. Synthesis and characterisation of a new low-spin iron(III) complex with 1,2-di(o-aminophenylthio)ethane and iron(III), cobalt(III) and manganese(III) complexes of 1,2-di(o-salicylaldiminophenylthio)ethane. Transition Met Chem 13, 58–63 (1988). https://doi.org/10.1007/BF01041501
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01041501