Abstract
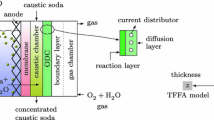
In order to explore the dependence of cell voltage on electrode geometries, precious metal oxide anodes (DSA®) with the following three kinds of geometries were constructed: an assembled strip with a parallel array (louvre type), a circularly perforated plate and a flattened mesh. Voltages in the membrane cell of a laboratory scale were measured with chlorine gas evolution in NaCl solution. The cell voltages exhibited a linear relation to a unit cell characteristic dimension as well as to the square of the per cent open area of the anode, as predicted from the theoretical analysis of the primary current distribution in the two-dimensional rectangular model cell. Therefore, small sizes of the iterative pattern unit reduced cell voltages. Slopes of the linear relation gave the increase in solution resistivity caused by the gas bubbles, indicating the presence of a bubble curtain around the anode.
Similar content being viewed by others
Abbreviations
- d 1 :
-
distance between the anode and the membrane
- d 2 :
-
thickness of the membrane
- j :
-
averaged current density, i.e. the current divided by (width of the anode)x(height of the anode)
- o p :
-
per cent open area defined by Equation 2
- p :
-
pitch of the unit region
- p cp :
-
pitch of the unit region for the circularly perforated anode
- p L :
-
pitch of the unit region for the louvered anode
- p LW,p SW :
-
pitch of the unit region for the mesh anode (see Fig. 2c)
- r :
-
radius of the circular open part
- V :
-
cell voltage of the whole cell
- V rs :
-
residual voltage, i.e. the sum of the voltages such as overpotential due to electrode kineties both at the anode and the cathode, ohmic drop in the catholyte, membrane potential drop and ohmic drop within both electrodes
- ϱ bc :
-
apparent resistivity of the anolyte containing gas bubbles
- ϱ 2 :
-
resistivity of the membrane
- θ:
-
arctan (p SW/p LW)
References
Y. Nishiki, K. Aoki, K. Tokuda and H. Matsuda,J. Appl. Electrochem. 14 (1984) 653.
Y. Nishiki, K. Aoki, K. Tokuda and H. Matsuda,J. Appl. Electrochem. 16 (1986) 291.
Y. Nishiki, K. Aoki, K. Tokuda and H. Matsuda,J. Appl. Electrochem. 17 (1987) 67.
L. E. Vaaler,J. Appl. Electrochem. 9 (1978) 21.
J. Jorne and J. F. Louvar,J. Electrochem. Soc. 127 (1980) 298.
F. Hine, M. Yasuda, M. Watanabe and M. Kurata,Soda to Enso (Soda and Chlorine) 7 (1981) 281.
F. Hine and K. Murakami,J. Electrochem. Soc. 128 (1981) 64.
L. J. J. Janssen, J. J. M. Geraets, E. Barendrecht and S. D. J. Van Stralen,Electrochim. Acta 27 (1982) 1207.
F. Hine, M. Tasuda, Y. Ogata and K. Hara,J. Electrochem. Soc. 131 (1984) 83.
C. Elsner and F. Coeuret,J. Appl. Electrochem. 15 (1985) 567.
Y. Nishiki, K. Aoki, K. Tokuda and H. Matsuda,J. Appl. Electrochem. 17 (1987) 445.
K. Aoki, Y. Nishiki, K. Tokuda and H. Matsuda.J. Appl. Electrochem. 17 (1987) 552.
R. J. Horvath, in ‘Modern Chlor-Alkali Technology’ (edited by K. Wall), Ellis Horwood, New York (1986) Vol. 3, Ch. 17.
O. Lanzi, R. F. Savinell and R. E. Horn,J. Appl. Electrochem. 14 (1984) 425.
K. Aoki, Y. Nishiki, K. Tokuda and H. Matsuda,Denkikagaku (J. Electrochem. Soc. Jpn) 55, (1987) 34.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nishiki, Y., Nakamatsu, S., Aoki, K. et al. Estimation of optimum anode geometry in chlor-alkali membrane cells. J Appl Electrochem 19, 90–94 (1989). https://doi.org/10.1007/BF01039395
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01039395