Summary
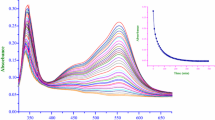
We propose the following rate law for the title anation reaction
within the 3.2 to 4.5 pH range, wherek a is the anation rate constant, andK E the ion-pair equilibrium constant between the substrate complex ion [Co(en)2(H2O)2]3+ and incoming zwitterionic ligand serine (Ser.H). Reaction rates in different ethanol-water mixtures reach a limit at high ligand concentrations and theK E increases as the concentration of the organic component in the medium increases, whereas reverse is true fork a. The reaction is catalysed by NO −3 ion and the rate increases with the increase of pH within the quoted range. ΔH ± and ΔS ± values were obtained from an Eyring plot and are compared with data for the isotopic water exchange process. A mechanism involving ion-pair formation between two reacting species, followed by dissociative interchange of the outer-sphere complex with the product is proposed.
Similar content being viewed by others
References
C. G. Barraclough and R. S. Murray,J. Chem. Soc., 7047 (1967).
R. Van Eldik and G. M. Harris,Inorg. Chem.,18, 1997 (1979).
D. R. Stranks and N. Vanderkoek,Inorg. Chem.,15, 2645 (1976).
K. Dutta and G. S. De,Ind. J. Chem. Sect. A,21, 477 (1982).
D. Chatterjee and G. S. De,J. Ind. Chem. Soc.,7, 526 (1985).
D. Chatterjee, K. Dutta and G. S. De,Ind. J. Chem.,25A, 350 (1986).
A. Ghoshal and S. K. Siddhanta,Ind. J. Chem.,20A, 661 (1981).
V. V. Udovenko, L. G. Reiter and E. P. Shkurman,Russ. J. Inorg. Chem.,6, 838 (1973).
M. N. Bishnu, B. Chakravarti, R. N. Banerjee and D. Banerjea,J. Coord. Chem.,13, 63 (1983).
F. P. Dwyer, A. M. Sergeson and L. K. Reid,J. Am. Chem. Soc.,85, 1219 (1963).
R. D. Gillard and G. Wilkinson,J. Chem. Soc., 3193 (1963).
Stability constants of Inorganic and Organic Ligands, London Chemical Society, p. 400.
J. Bjerrum and S. E. Rasmussen,Acta Chem. Scand.,6, 1265 (1952).
W. Kruse and H. Taube,J. Am. Chem. Soc.,83, 1280 (1961).
S. Glasstone,Text Book of Physical Chemistry, MacMillan 1977, p. 1116.
F. Basolo and R. G. Pearson,Mechanism of Inorganic Reactions, Wiley Eastern, New Delhi, 1977, p. 154.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chatterjee, D., De Sankar, G. Kinetics and mechanism of anation ofcis-diaquo-bis-ethylenediamine cobalt(III) ion by L-serine in ethanol-water mixtures of varying dielectric constant. Transition Met Chem 12, 193–196 (1987). https://doi.org/10.1007/BF01023526
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01023526