Abstract
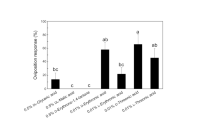
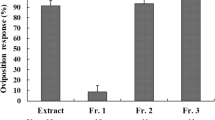
Β-Bergamotenoic acid, a compound previously shown to stimulate oviposition inH. zea, was converted into a set of bicyclic analogs and tested with a set of acyclic side chain analogs to ascertain the molecular structure that maximizes insect behavioral response. While changes in the bicyclic ring elicited no variation in response, alteration in the side chain structure ofΒ-bergamotenoic acid resulted in significant changes in moth preference. Free rotation about the C-C bond proximal to the carboxylic acid group appears to be an important structural factor, since saturation of the side chain double bond significantly increased activity. The carboxylic acid group seems to be required for strong oviposition stimulation, since analogs lacking the carboxylic acid group exhibited no significant oviposition activity. Oviposition preference ofH. zea was also influenced by the length of the hydrocarbon chain to which the carboxylic acid is attached. While hexanoic acid was found inactive, the ovipositional preference for the heptanoic and octanoic acids was greatest for the one 8-carbon tested. This and other work suggest that carboxylic acids of specific chain lengths influence the oviposition behavior of bothHelicoverpa andHeliothis species and may be associated with host-plant selection. The potential use of this information in designing integrated pest management strategies for control ofH. zea is discussed.
Similar content being viewed by others
References
Bengtsson, M., Liljefors, T., Hansson, B.S., Lofstedt, C., andCopaja, S.V. 1990. Structure-activity relationships for chain-shortened analogs of (Z)-5-decenyl acetate, a pheromone component of the turnip moth,Agrotis segetum.J. Chem. Ecol. 16:667–684.
Cantelo, W.W., andJacobson, M. 1979. Corn silk volatiles attract many species of moths.J. Environ. Sci. Health 14:695–707.
Caine, D. 1976. Reduction and related reactions ofα, Β-unsaturated carbonyl compounds with metals in liquid ammonia.Org. React. 23:1–258.
Coates, R.M., Ley, D.A., andCavender, P.L. 1978. Synthesis and carbon-13 nuclear magnetic resonance spectra of all-trans-geranylgeraniol and its Nor analogues.J. Org. Chem. 43:4915.
Coates, R.M., Dennisen, J.F., Juvik, J.A., andBabka, B.A. 1988. Identification ofα-santalenoic and endo-Β-bergamotenoic acids as moth oviposition stimulants from wild tomato leaves.J. Org. Chem. 53:2186.
DeBore, T.J., andBacker, H.J. 1963. Diazomethane.Org. Synth. Coll. Vol. 4:250–253.
Dobner, B., Elsner, B., andNuhn, P. 1989. Synthese mittelstÄndig verzweigter FettsÄuren.Pharmazie 44:758.
Dorough, G.L., Glass, H.B., Gresham, T.L., Malone, G.B., andReid, E.E. 1941. Structureproperty relationships in some isomeric octanols.J. Am. Chem. Soc. 63:3100.
Evershed, R.P. 1988. Insect olfaction and molecular structure, pp. 1–33,in D.E. Morgan, and N.B. Mandava (eds.). CRC Handbook of Natural Pesticides. Vol IV, Part A. Academic Press, New York.
Fortunato, J.M., andGanem, B. 1976. Lithium and potassium trialkylborohydrides. Reagents for direct reduction ofα,Β-unsaturated carbonyl compounds to synthetically versatile enolate anions.J. Org. Chem. 41:2194.
Goffreda, J.C., Mutschler, M.A., Ave, D.A., Tingey, W.M., andSteffens, J.C. 1989. Aphid deterrence by glucose esters in glandular trichome exudate of the wild tomatoLycopersicon pennellii.J. Chem. Ecol. 15:2135–2147.
Goodnight, J.H. 1982. ANOVA, pp. 113–137,in SAS User's Guide: Statistics. SAS Institute, Cary, North Carolina.
Johnson, S.J.,King, E.G., andBradley, J.R. 1986. Theory and tactics ofHeliothis control.S. Coop. Ser. Bull. 316:161 pp.
Jones, R.L., Burton, R.L., McGovern, T.P., andBerosa, M. 1973. Potential oviposition inducers for corn earworms.Environ. Entomol. 66:921–925.
Juvik, J.A., Berlinger, M.J., Ben-David, T., andRudich, J. 1982. Resistance among accessions of the genusLycopersicon andSolanum to four of the major insect pests of tomatoes in Israel.Phytoparasitica 10:145–156.
Juvik, J.A., Babka, B.A., andTimmermann, E.A. 1988. Influence of trichome exudates from species of Lycopersicon on oviposition behavior ofHeliothis zea (Boddie).J. Chem. Ecol. 14:1261.
Kawashima, M., Sato, T., andFujisawa, T. 1989. A facile method for synthesis of three carbonhomologated carboxylic acid by regioselective ring-opening ofΒ-propiolactones with organocopper reagents.Tetrahedron 45:403.
Kogan, J., Sell, D.K., Stinner, R.E., Bradley, J.R., Jr., andKogan, M. 1978. A bibliography ofHeliothis zea andHeliothis virescens.Intsoy 17:1–8.
Leonard, B.R., Graves, J.B., Burris, E., Pavloff, A.M., andChurch, G. 1989.Heliothis spp. (Lepidoptera: Noctuidae) captures in pheromone traps: Species composition and relationship to oviposition in cotton.J. Econ. Entomol. 82:574–579.
Metcalf, R.L., Mitchell, W.C., andMetcalf, E.R. 1983. Olfactory receptors in the melon fly,Dacus cucurbitae and the oriental fruit flyDacus dorsalis.Proc. Natl. Acad. Sci. U.S.A. 80:3143–3147.
Metcalf, R.L., Metcalf, E.R., andMitchell, W.C. 1986. Benzyl acetates as attractants for the male oriental fruit flyDacus dorsalis, and the male melon fly,Dacus cucurbitae.Proc. Natl. Acad. Sci. U.S.A. 78:4007–4010.
Micovic, V.C., andMihailovic, M.L.J. 1953. The reduction of acid amides with lithium aluminum hydride.J. Org. Chem. 18:1190.
Moncrieff, R.W. 1967. The Chemical Senses, 3rd ed. Leonard Hill, London.
Raulston, J.R., Summy, K.R., Loera, J., Puir, S.D., andSparks, A.N. 1990. Population dynamics of corn earworm larvae (Lepidoptera: Noctuidae) on corn in the lower Rio Grande Valley.Envirn. Entomol. 19:274–280.
Rick, C.M. 1973. Potential genetic resources in tomato species; clues from observations in native habitats, pp. 255–269,in A. Hollaender et al. (eds.). Genes, Enzymes, and Populations. Plenum Press, New York.
Severson, R.F., Jackson, D.M., Johnson, A.W., Sisson, V.A., andStephenson, M.G. 1991. Ovipositional behavior of tobacco budworm and tobacco hornworm, pp. 265–278,in P.A. Hedin (ed.). Naturally Occurring Pest Bioregulators. American Chemical Society, Washington, D.C.
Sinha, N., andJuvik, J.A. 1987. Chemical factors associated with resistance toHeliothis zea in wild accessions of tomatoes.HortSci. 22:1105.
Still, W.C., Kahn, M., andMitra, A. 1978. Rapid chromatographic technique for preparative separations with moderate resolution.J. Org. Chem. 43(14):2923–2925.
Tai, A., Matsumura, F., andCoppel, H.C. 1971. Synthetic analogues of the termite trail following pheromone, structure and biological activity.J. Insect Physiol. 17:181.
University of California. 1985. Integrated pest management for tomatoes. Division of Agriculture and Natural Resources. Pub. 3274. 104 pp.
Walters, D.S., andSteffens, J.C. 1990. Branched chain amino acid metabolism in the biosynthesis ofLycopersicon pennellii glucose esters.Plant Physiol. 93:1544–1551.
White, J.D., Takabe, K., andPrisbylla, M.P. 1985. Steroselective synthesis of trisporic acids A and B, their methyl esters, and trisporols A and B, hormones and prohormones of mucoraceous fungi.J. Org. Chem. 50:5233–5244.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Douglass, S.K., Juvik, J.A., Pyun, HJ. et al. Structure-activity relationships for analogs of (+)-(E)-endo-Β-bergamoten-12-oic acid, an oviposition stimulant ofHelicoverpa zea (Boddie). J Chem Ecol 19, 11–27 (1993). https://doi.org/10.1007/BF00987467
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00987467