Abstract
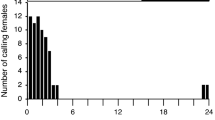
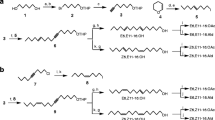
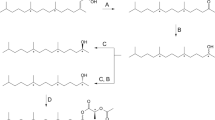
(R)-6-Ethyl-2-methyl-2,3-dihydro-4H-pyran-4-one, (1R,3S,5R)-3-ethyl-1,8-dimethyl-2,9-dioxabicyclo[3.3. 1]non-7-ene, and (1R,3S,5R)-3-ethyl-1,8-dimethyl-2,9-dioxabicyclo[3.3.1]non-7-en-6-one represent the main components in the male pheromone of the swift moth,Hepialus hecta. The amounts of the three components were 40, 5, and 5 μg per male, respectively. Structure elucidation of the compounds was based on spectroscopic data as compared to synthetic reference samples. The absolute configurations were determined by gas chromatography on chiral stationary phases; optically active samples served as reference compounds. Electrophysiological and behavioral experiments with natural material and synthetic samples clearly showed the three heterocyclic compounds to act as pheromones. (E, E)-α-Farnesene represents the main component of the scent secretion of maleHepialus humuli.
Similar content being viewed by others
References
Bertkau, P. 1882. Ueber den Duftapparat vonHepialus hectusL.Arch. Naturgesch. 17:223–224.
Boppré, M. 1984. Chemically medicated interactions between butterflies, pp. 259–275in R.I. Vane-Wright, and P.R. Ackery (eds.). The Biology of Butterflies. Academic Press, London.
Bopprë, M., andSchneider, D. 1989. The biology ofCreatonotos (Lepidoptera: Arctiidae) with special reference to the androconial system.Zool. J. Linn. Soc. 96:339–356.
Deshong, S., Ramesh, S., Elango, V., andPerez, J.J. 1985. Total synthesis of (±)-triandamycin A.J. Am Chem. Soc. 107:5219–5224.
Francke, W., Mackenroth, W., Schröder, W., Schultz, S., Tengö, J., Engel, E., Engels, W., Kittmann, R., andSchneider, D. 1985. Identification of cyclic enolethers from insects: Alkyldihydropyranes from bees and alkyldihydro-4H-pyran-4-ones from male moth.Naturwissenschaften 40c:145–147.
Francke, W., Schulz, S., Sinnwell, A., König, W.A., andRoisin, Y. 1989. Epoxytetrahydroedulan, a new terpenoid from the hairpencils ofEuploea (Lep.: Danainae) butterflies.Liebigs Ann. Chem. 1989:1195–1201.
Gelin, S., andGelin, R. 1968. Synthéses et propriétés de dihydropyrones-4 et de β-dicétones éthyléniques.Bull. Soc. Chim. Fr. pp. 288–298.
Georgian, V., Harrisson, R., andGubisch, N. 1959. A new chemical desulfurization method.J. Am. Chem. Soc. 81:5834–5835.
Kaissling, K.E., andThorson, J. 1980. pp. 261–282,in D.B. Satelle, et al. (eds.) Receptors for Neurotransmitters, Hormones and Pheromones in Insects. Elsevier/North Holland Biomedical Press.
König, W.A., Benecke, I., andBretting, H. 1981. Gaschromatographische Trennung enantiomerer Kohlenhydrate an einer neuen chiralen stationären Phase.Angew. Chem. 93:688–690.
Kubo, I., Matsumoto, T., andWagner, D.L. 1985. Isolation and structure of hepialone; principle component from male sex scales of (H. californiens (Lepidoptera).Tetrahedron Lett. 26:563–566.
Mallet, J. 1984. Sex roles in the ghost mothHepialus humuli (L.) and a review of mating in the Hepialidae (Lepidoptera).Zool. J. Linn. Soc. 79:67–82.
Mori, K., andKisida, H. 1986. Synthesis of both enantiomers of the heterocyclic pheromones isolated from the male swift mothH. hectaL.Tetrahedron 42:5281–5290.
Rice, K.C. andDyer, J.R. 1975. A practical synthesis of 2,3-dimethylfuran and an efficient stereoselective preparation of (Z)-3-methyl-2-penten-1,4-diol.J. Heterocycl. Chem. 12:1325–1326.
Schneider, D. 1984. Pheromone biology of the Lepidoptera: overview, some recent findings and some generalizations, pp. 301–313,in L. Bolis, R.D. Keynes, and S.H.P. Maddrell (eds.). Comparative Physiology of Sensory Systems. Cambridge University Press, Cambridge, U.K.
Schurig, V., andWistuba, D. 1984. Analytical enantiomer separation of aliphatic diols as boronates and acetals by complexation gas chromatography.Tetrahedron Lett. 25:5633–5636.
Sinnwell, V., Schulz, S., Francke, W., Kittmann, R., andSchneider, D. 1985. Identification of pheromones from the male swift mothHepialus hectaL.Tetrahedron Lett. 26:1707–1710.
Turner, J.R.G. 1976. Sexual behaviour: female swift moth is not the aggressive partner.Anim. Behav. 24:188–190.
Turner, J.R.G. 1988. Sex, leks and fechts in swift mothsHepialus (Lepidoptera, Hepialidae): Evidence for the hot shot moth.Entomologist 102:90–95.
Wunderer, H., Hansen, K., Bell, T.W., Schneider, D., andMeinwald, J. 1985. Sex pheromones of two Asian moths (Creatonotos tansiens, C. gangis, Lepidoptera-Arctiidae): Behavior, morphology, chemistry and electrophysiology.Exp. Biol. 46:11–27.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Schulz, S., Francke, W., König, W.A. et al. Male pheromone of swift moth,Hepialus hecta L. (Lepidoptera: Hepialidae). J Chem Ecol 16, 3511–3521 (1990). https://doi.org/10.1007/BF00982114
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00982114