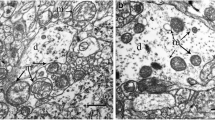
Abstract
Synaptosomes were isolated from the motor area of the cerebral cortex of normoxic or hypoxic (PaO2=17–19 mmHg, for 15 min) beagle dogs of different ages. Synaptosomes were incubated in Krebs-Henseleit-Hepes buffer (for 10 min at 24°C) and the energetic state was defined by: the balance of the labile phosphates (ATP, ADP, AMP, and creatine phosphate); the respiratory rate; the redox state of the intramitochondrial NAD-couple. By the present experimental model, it is possible to evaluate the potential damage (induced by the “in vivo” hypoxic insult) that synaptosomes cannot reverse under optimal incubation. Aging affected the phosphorylation state of the post-hypoxic incubated synaptosomes. The oxygen consumption rate was quite similar in the synaptosomal fractions from the motor area of hypoxic beagle dogs of different ages, but the cytochromec anda contents were lower in the preparations from hypoxic older brains. In dogs of different ages, hypoxia always lowered the respiration of the synaptosomes, but aging affected the oxygen consumption rates only in post-hypoxic synaptosomes incubated with succinate. The synaptosomal energetic state was defined also by the redox state of the intramitochondrial NAD-couple (ΔGox-red) and the phosphorylation state of adenine nucleotide system (ΔGATP). The free-energy change (ΔΔG) for the coupled reactions was calculated, too. In synaptosomes isolated from the cerebral cortex of dogs submitted to hypoxia, the equilibrium (calculated for the mitochondrial electron transfer chain and the phosphorylation of adenine nucleotides) was markedly altered as function of aging. The extensive age-related ΔΔG changes were largely supported by alteration of the phosphorylation state of adenine nucleotides, rather than by modification of the redox state of the electron transfer chain.
All present data suggest that the bioenergetic derangement caused by hypoxia and aging may be interpreted also in terms of modification of the biophysical and biochemical mechanisms involving the mitochondrial membranes and particularly the inner mitochondrial membrane.
Similar content being viewed by others
References
Bachelard, H. S., Lewis, L. D., Pontén, V., and Siesjö, B. K. 1974. Mechanisms activating glycolysis in the brain in arterial hypoxia. J. Neurochem. 22:395–401.
Bartlett, M. S. 1937. Properties of sufficiency and statistical tests. Proc. Roy. Soc. 160:268–279.
Benzi, G., Arrigoni, E., Manzo, L., De Bernardi, M., Ferrara, A., Panceri, P., and Berte', F. 1973. Estimation of changes induced by drugs in cerebral energy-coupling processes “in situ” in the dog. J. Pharm. Sci. 62:758–764.
Benzi, G., Arrigoni, E., Dagani, F., Pastoris, O., Villa, R. F., and Agnoli, A. 1978. Cerebral energy state during or after hypoxia and complete or incomplete ischemia. J. Appl. Physiol. 45:312–319.
Benzi, G., Arrigoni, E., Agnoli, A., Raimondo, S., Fulle, D., Pastoris, O., Curti, D., and Villa, R. F. 1982. Influence of age upon cerebral metabolic changes induced by acute hypoxia on the synaptosomes from dog brain. Exp. Geront. 17:19–32.
Benzi, G., Arrigoni, E., Pastoris, O., Villa, R. F., Dossena, M., Agnoli, A., and Giuffrida, A. M. 1982. Drug action on the metabolic changes induced by acute hypoxia on synaptosomes from the cerebral cortex. J. CBF metab. 1:229–239.
Benzi, G., and Villa, R. F. 1976. Adenyl cyclase system and cerebral energy state. J. Neurol. Neurosurg. Psychiatry 39:77–83.
Bernt, E., Bergmeyer, H. U., and Möllering, H. 1974. Creatine. Vol. 4, pages 1772–1776, in Bergmeyer, H. U. (ed.), Methods of Enzymatic Analysis, Verlag Chemie/Academic Press, New York, London.
Booth, R. F., and Clark, J. B. 1978. A rapid method for the preparation of relatively pure, metabolically competent synaptosomes from rat brain. Biochem. J. 176:365–370.
Bradford, H. F. 1969. Respiration in vitro of synaptosomes from mammalian cerebral cortex. J. Neurochem. 16:675–684.
Czok, R., and Lamprecht, W. 1974. Pyruvate, phosphoenolpyruvate andd-glycerate-2-phosphate. Vol. 3, pages 1446–1451, in Bergmeyer, H. U. (ed.). Methods of Enzymatic Analysis, Verlag Chemie/Academic Press, New York, London.
De Robertis, E., Pellegrino de Iraldi, A., de Lores Arnaiz, G. R., and Salganicoff, L. 1962. Cholinergic and non-cholinergic nerve endings in rat brain. J. Neurochem. 9:23–35.
Dutton, P. L., and Wilson, D. F. 1974. Redox potentiometry in mitochondrial photosynthetic bioenergetics. Biochim. Biophys. Acta 346:165–212.
Erecinska, M., and Wilson, D. F. 1978. Homeostatic regulation of cellular energy metabolism. TIBS 3:219–223.
Erecinska, M., Kula, T., and Wilson, D. F. 1978. Regulation of energy metabolism. Evidence against a primary role of adenine nucleotide translocase. FEBS Lett. 87:139–144.
Erecinska, M., Wilson, D. F., and Nishiki, K. 1978. Homeostatic regulation of cellular energy metabolism. Experimental characterization and fit to a model. Am. J. Physiol. 234:C82-C89.
Folbergrova', J., MacMillan, V., and Siesjö, B. K. 1972. The effect of moderate and marked hypercapnia upon the energy state and upon the cytoplasmatic NADH/NAD+ ratio of the rat brain. J. Neurochem. 19:2497–2505.
Folbergrova', J., MacMillan, V., and Siesjö, B. K. 1972. The effect of hypercapnic acidosis upon some glycolytic and Krebs cycle-associated intermediates in the rat brain. J. Neurochem. 19:2507–2517.
Gray, E. G., and Whittaker, V. P. 1962. The isolation of nerve endings from brain. An electron-microscopic study of cell fragments derived by homogenization and centrifugation. J. Anat. 26:79–87.
Grossman, R. G., and Williams, V. F. 1971. Electrical activity and ultrastructure of cortical neurones and synapses in ischemia. Pages 61–75, in Meldrum (ed.), Brain Hypoxia, Spastic International Medical Publications, Lippincott, Philadelphia.
Gutmann, I., and Wahlefeld, A. W. 1974. L-(+)-Lactater determination with lactate dehydrogenase and NAD. Vol. 3, pages 1464–1468, in Bergmeyer, H. U. (ed.), Methods of Enzymatic Analysis, Verlag Chemie/Academic Press, New York, London.
Hess, H. H., and Derr, J. E. 1975. Assay of inorganic and organic phosphorus in the 0.1–5 nanomole range. Anal. Biochem. 63:607–613.
Jaworek, D., Gruber, W., and Bergmeyer, H. U. 1974. Adenosine-5′-disphosphate and adenosine-5′-monophosphate. Vol. 4, pages 2127–2131, in Bergmeyer, H. U. (ed.), Methods of Enzymatic Analysis, Verlag Chemie/Academic Press, New York, London.
Krebs, H. A., Mellanby, J., and Williamson, D. H. 1962. The equilibrium constant of the 3-hydroxybutyric dehydrogenase system. Biochem. J. 82:96–98.
Lamprecht, W., and Trautschold, I. 1974. ATP: determination with hexokinase and glucose-6-phosphate dehydrogenase. Vol. 4, pages 2101–2110, in Bergmeyer, H. U. (ed.), Methods of Enzymatic Analysis, Verlag Chemie/Academic Press, New York, London.
Lamprecht, W., Stein, P., Heinz, F., and Weisser, H. 1974. Creatine phosphate. Vol. 4, pages 1777–1781, in Bergmeyer, H. U. (ed.), Methods of Enzymatic Analysis, Verlag Chemie/Academic Press, New York, London.
Lowry, O. H., Rosebrough, M. J., Farr, A. L., and Randall, R. J. 1951. Protein measurement with the folin phenoi reagent. J. Biol. Chem. 193:265–275.
Nohl, H. 1979. Influence of age on thermotropic kinetics of enzymes involved in mitochondrial energy metabolism. Z. Geront. 12:9–18.
Nohl, H., and Krämer, R. 1980. Molecular basis of age-dependent changes in the activity of adenine nucleotide translocase. Mech. Age. Dev. 14:137–144.
Nohl, H. 1982. Age-dependent changes in the structure-function correlation of ADP/ATP-translocating mitochondrial membranes. Gerontology 28:354–359.
Rafalowska, U., Erecinska, M., and Wilson, D. F. 1980. Energy metabolism in rat brain synaptosomes from nembutalanesthetized and nonanesthetized animals. J. Neurochem. 34:1380–1386.
Scherer, B., and Klingenberg, M. 1974. Demonstration of the relationship between the adenine nucleotide carrier and the structural changes of mitochondria as induced by adenosine-5′-diphosphate. Biochemistry 13:161–170.
Siesjö, B. K. 1978. Nitrous oxide. Pages 242–244, in Siesjö, B. K. (ed.), Brain Energy Metabolism, J. Wiley & Sons, Chichester.
Siesjö, B. K., and Nilsson, L. 1971. The influence of arterial hypoxemia upon labile phosphates and upon extracellular and intracellular lactate and pyruvate concentration in the rat brain. Scand. J. Clin. Lab. Invest. 27:83–96.
Siesjö, B. K., and Zwetnow, N. N. 1970. The effect of hypovolemic hypotension on extra- and intra-cellular acid-base parameters and energy metabolites in the rat brain. Acta Physiol. Scand. 79:114–124.
Silver, I. A. 1973. Local PO2 in relation to intracellular pH, cell membrane potential and potassium leakage in hypoxia and shock. Adv. Exp. Biol. Med. 37A:223–231.
Veech, R. L., Harris, R. L., Veloso, D., and Veech, E. H. 1973. Freeze-blowing: a new technique for the study of brain in vivo. J. Neurochem. 20:183–188.
Williamson, D. H., Mellanby, J., and Krebs, H. A. 1962. Enzymatic determination of D-(−)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem. J. 82:90–96.
Williamson, D. H., Lund, P., and Krebs, H. A. 1967. The redox state of the free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem. J. 103:514–527.
Wilson, D. F., Stubbs, M., Oshino, N., and Erecinska, M. 1974. Thermodynamic relationships between the mitochondrial oxidation-reduction reactions and cellular ATP-levels in ascites tumor cells and perfused rat liver. Biochemistry 13:5305–5311.
Wilson, D. F., Stubbs, M., Veech, R. L., Erecinska, M., and Krebs, H. A. 1974. Equilibrium relations between the oxidation-reduction reactions and the adenosine triphosphate synthesis in suspensions of isolated liver cells. Biochem. J. 140:57–64.
Wilson, D. F., Owen, C. S., and Holian, A. 1977. Control of mitochondrial respiration. A quantitative evaluation of the roles of cytochrome c and oxygen. Arch. Biochem. Biophys. 182:749–762.
Author information
Authors and Affiliations
Additional information
Special Issue Dedicated to Dr. Abel Lajtha.
Rights and permissions
About this article
Cite this article
Benzi, G., Giuffrida, A.M. Changes of synaptosomal energy metabolism induced by hypoxia during aging. Neurochem Res 12, 149–157 (1987). https://doi.org/10.1007/BF00979531
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00979531