Abstract
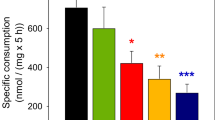
Oxidative decarboxylation of [1-14C]pyruvate was studied in primary cultures of neurons and of astrocytes. The rate of this process, which is a measure of carbon flow into the tricarboxylic acid (TCA) cycle and which is inhibited by its end product, acetyl CoA, was determined under conditions which would either elevate or reduce the components of the malate-aspartate shuttle (MAS). Addition of aspartate (1 mM) was found to stimulate pyruvate decarboxylation in astrocytes whereas addition of glutamate (or glutamine) had no effect. Since aspartate is a precursor for extramitochondrial malate, and thus intramitochondrial oxaloacetate, whereas glutamate and glutamine are not, this suggests that an increase in oxaloacetate level stimulates TCA cycle activity. Conversely, a reduction of the glutamate content by 3 mM ammonia, which might reduce exchange between glutamate and aspartate across the mitochondrial membrane, suppressed pyruvate decarboxylation. This effect was abolished by addition of glutamate or glutamine or exposure to methionine sulfoximine (MSO). These findings suggest that impairment of MAS activity by removal of MAS constituents decreases TCA cycle activity whereas replenishment of these compounds restores the activity of the TCA cycle. No corresponding effects were observed in neurons.
Similar content being viewed by others

References
Hertz, L., Murthy, Ch. R. K., Lai, J. C. K., Fitzpatrick, S. M., and Cooper, A. J. L. 1987. Some metabolic effects of ammonia on astrocytes and neurons in primary cultures. Neurochem. Pathol., 6:97–129.
Siess, E., Wittman, J., and Wieland, O. 1971. Interconversion and kinetic properties of pyruvate dehydrogenase from brain. Hoppe Seylers Z. Physiol. Chem. 352:447–452.
Hindfelt, B., Plum, F., and Duffy, T. E. 1977. Effect of acute ammonia intoxication on cerebral metabolism in rats with portacaval shunts. J. Clin. Invest 59:386–396.
Duffy, T. E., and Plum, F. 1982. Hepatic Encephalopathy, in The Liver: Biology and Pathobiology Pages 693–715,in Arias, I., Poppor, D., Schachter, D., and Shafritz, D. A. (eds.). Raven Press, New York.
Fitzpatrick, S. M., Cooper, A. J. L., and Duffy, T. E. 1983. Use of β-methyleneaspartate to assess the role of aspartate aminotransferase in cerebral oxidative metabolism. J. Neurochem. 41:1370–1383.
Fitzpatrick, S. M. 1984. Role of Aspartate Aminotransferase in Cerebral Oxidative Metabolism. Thesis, Cornell University.
Hertz, L., Yu, A. C. H., Potter, R. L., Fisher, T. E., and Schousboe, A. 1983. Metabolic fluxes from glutamate and towards glutamate in neurons and astrocytes in primary cultures. Pages 327–342,in Hertz, L., Kvamme, E., McGeer, E. G., and Schousboe, A. (eds.), Glutamine, Glutamate and GABA in the Central Nervous System. Alan R. Liss, New York.
Kvamme, E., Svenneby, G., Hertz, L., and Schousboe, A. 1982. Properties of phosphate activated glutaminase in astrocytes cultured from mouse brain. Neurochem. Res. 7:761–770.
Drejer, J., Benveniste, H., Diemer, N. M., and Schousboe, A. 1985. The cellular origin of ischemia-induced glutamate release from brain tissue in vivo and in vitro. J. Neurochem. 45:145–151.
Hertz, L., Juurlink, B. H. J., and Szuchet, S. 1985. Cell cultures. Pages 603–661,in Lajtha A. (ed.), Handbook of Neurochemistry, 2nd Ed., Vol. 8. Plenum Press, New York.
Hertz, L., Juurlink, B. H. J., Szuchet, S., and Walz, W. 1985. Cell and tissue cultures. Pages 117–167,in Boulton, A. A. and Baker, G. B. (eds.), Neuromethods: General Neurochemical Methods, Vol. 1, Humana Press, Clifton, New Jersey.
Hertz, L., Juurlink, B. H. J., Hertz, E., Fosmark, H., and Schousboe, A. 1987. Preparation of primary cultures of mouse (rat) astrocytes,in Shahar, A., Filogamo, G., and Haber, B. (eds.), A Dissection and Tissue Culture Manual for the Nervous System. Alan R. Liss, New York, in press.
Hertz, E., Yu, A. C. H., Hertz, L., Juurlink, B. H. J., and Schousboe, A. 1987. Preparation of primary cultures of mouse cortical neurons,in Shahar, A., Filogamo, G., and Haber, B. (eds.), A Dissection and Tissue Culture Manual for the Nervous System. alan R. Liss, New York, in press.
Hertz, L., Juurlink, B. H. J., Fosmark, H., and Schousboe, A. 1982. Astrocytes in primary cultures. Pages 157–174,in Pfeiffer, S. E. (ed.), Neuroscience Approached Through Cell Culture. CRC Press, Boca Raton, Florida.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. 1951. Protein measurement with Folin phenol reagent. J. Biol. Chem. 193:265–275.
Harper, J. F. 1984. Peritz' F test: Basic program of a robust multiple comparison test for statistical analysis of all differences among group means. Comput. Biol. Med. 14:D437–445.
Nicklas, W. J., Clark, J. B., and Williamson, J. R. 1971. Metabolism of rat brain mitochondria. Studies on the potassium ion-stimulated oxidation of pyruvate. Biochem. J. 123:83–95.
Roche, T. E., and Reed, L. J. 1974. Monovalent cation requirement for ADP inhibition of pyruvate dehydrogenase kinase. Biochem. Biophys. Res. Commun. 59:1341–1348.
Beck, D. P., Broyles, J. L., and Von Korff, R. W. 1977. Role of malate transport in regulating metabolism in mitochondria isolated from rabbit brains. J. Neurochem. 29:487–493.
McKhann, G. M., and Tower, D. B. 1961. Ammonia toxicity and cerebral oxidative metabolism. Am. J. Physiol. 200:420–424.
Minn, A., and Gayet, J. 1977. Kinetic study of glutamate transport in rat brain mitochondria. J. Neurochem. 29:873–881.
Hertz, L., and Schousboe, A. 1986. Role of astrocytes in compartmentation of amino acid and energy metabolism. Pages 179–208,in Fedoroff, S. and Vernadakis, A. (eds.), Astrocytes, Vol. 2. Academic Press, New York.
Norenberg, M. D., and Martinez-Hernandez, A. 1979. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 161:303–310.
Lajtha, A., Maker, H. S., and Clarke, D. D. 1981. Chapter 17, Metabolism and transport of carbohydrates and amino acids. Pages 329–353,in Siegel, G. J., Albers, W., Agranoff, B. W., and Katzman, R. (eds.), Basic Neurochemistry, 3rd Edition. Little, Brown and Company, Boston.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Murthy, C.R.K., Hertz, L. Pyruvate decarboxylation in astrocytes and in neurons in primary cultures in the presence and the absence of ammonia. Neurochem Res 13, 57–61 (1988). https://doi.org/10.1007/BF00971855
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00971855