Abstract
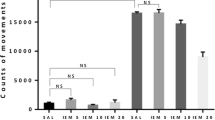
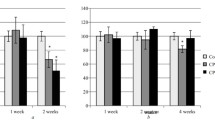
We tested the effect of glycine on phencyclidine (PCP)-induced hyperactivity in mice. Glycine antagonized the locomotor stimulating effect of PCP. Correlation was found between the degree of antagonistic effect and the size of the increase in glycine in the brain. The antagonism is not due to changes in uptake, since the elevation of glycine in plasma and brain had no effect on the cerebral uptake of PCP. This pharmacological action of glycine appears to be a central effect, but some peripheral effect can not be excluded. Since glycine is not toxic at levels needed for PCP antagonism, it could be considered for ameliorating PCP psychosis. The locomotor stimulating effect of PCP is strain dependent in mouse. Some strains are responsive, such as BALB/cBy and CXBK, and some are unresponsive, such as C57BL/6 and CXBH.
Similar content being viewed by others
References
Allen, R. M., Young, S. J. 1978. Phencyclidine-induced psychosis. Am. J. Psychiat. 135:1081–1084.
Castellani, S., andAdams, P. M. 1981. Acute and chronic phencyclidine effects on locomotor activity, stereotypy and ataxia in rats. Eur. J. Pharmacol. 73:143.
Lozovsky, D., Saller, C. F., Bayorh, M. A., Chiueh, C. C., Ric, K. C., Burke, T. R., andKopin, J. J., 1983. Effects of phencyclidine on rat prolactin, dopamine receptor and locomotor activity. Life Sci. 32:2725–2731.
Snyder, S. H. 1980. Phencyclidine. Nature 285:355–356.
Castellani, S., Giannini, J. A., andAdams, P. M. 1982. Physostigmine and haloperidol treatment of acute phencyclidine intoxication. Am. J. Psychiat. 139:508–510.
Freed, W. J., Weinberger, D. R., Bing, L. A., andWyatt, R. J. 1980. Neuropharmacological studies of phencyclidine (PCP)-induced behavioral stimulation in mice. Psychopharmacology 71:291–297.
Freed, W. J., Bing, L. A., andWyatt, R. J. 1984. Effects of neuroleptics on phencyclidine (PCP)-induced locomotor stimulation in mice. Neuropharmacology 23:175–181.
Chaturvedi, A. K. 1984. Effects of mecamylamine, nicotine, atropine and physostigmine on the phencyclidine-induced behavioral toxicity. Pharmacol. Biochem. Behav. 20:559–566.
Smith, C. R., Meltzer, H. Y., Arora, R. C., andDavis, M. J. 1977. Effects of phencyclidine on [3H]catecholamine and [3H]serotonin uptake in synaptosomal preparations from rat brain. Biochem. Pharmacol. 26:1435–1439.
Vickroy, W. T., andJohnson, K. M. 1980. In vivo administration of phencyclidine inhibits3H-dopamine accumulation by rat brain striatal slices. Subs. Alc. Act./Misuse 1:351–354.
Bagchi, S. P. 1981. Effect of phencyclidine on synaptosomal dopamine continuously appearing from phenylalanine: sensitivity to reserpine. Neuropharmacology 20:845–851.
Beninger, R. J. 1983 The role of dopamine in locomotor activity and learning. Brain Res. Rev. 6:173–196.
Cherany, A., Nieoullon, A., andGlowinsky, J. 1978. Inhibition of dopamine release in the cat caudate nucleus by nigral application of glycine. Eur. J. Pharmacol. 47:141–147.
Leviel, V., Cherany, A., Nieoullon, A., andGlowinsky, J. 1979. Symmetric bilateral changes in dopamine release from the caudate nuclei of the cat induced by unilateral nigral application of glycine and GABA-related compounds. Brain Res. 175:259–270.
Toth, E., Lajtha, A., Sarhan, S., andSeiler, N. 1983. Anticonvulsant effects of some inhibitory neurotransmitter amino acids. Neurochem. Res. 8:291–301.
Toth, E. andLajtha, A. 1981. Elevation of cerebral levels of nonessential amino acids in vivo by administration of large doses. Neurochem. Res. 6:1309–1317.
Toth, E. andLajtha, A. 1980. Effect of protein-free diet on the uptake of amino acids by brain in vivo. Exp. Neurol. 68:443–452.
Seversan, J. A., Randall, P. K., andFinch, C. E. 1981. Genotypic influences on striatal dopaminergic regulation in mice. Brain Res. 210:201–215.
Boehue, E. R. andCiaranello, D. R. 1982. Genetic control of dopamine and serotonin receptors in brain regions of inbred mice. Brain Res. 266:51–65.
Toth, E. andLajtha, A. 1984. Brain protein synthesis rates are not sensitive to elevated GABA, taurine or glycine. Neurochem. Res. 9:173–179.
Seiler, N., andSarhan, S. 1984. Synergistic anticonvulsant effects of GABA-T inhibitors and glycine. Naunyn-Schmiedeberg's Arch. Pharmacol. 311:179–184.
Pycock, C. J., andKerwin, R. W. 1981. The status of glycine as a supraspinal neurotransmitter. Life Sci. 28:2679–2686.
McGeer, P. L., Eccles, J. C., andMcGeer, E. G. 1978. Molecular Neurobiology of the Mammalian Brain. Page 229, Plenum Press, New York.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Toth, E., Lajtha, A. Antagonism of phencyclidine-induced hyperactivity by glycine in mice. Neurochem Res 11, 393–400 (1986). https://doi.org/10.1007/BF00965013
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00965013