Summary
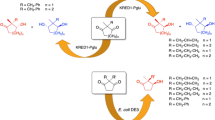
Twelve strains of Eumycetes were able to perform the reduction of 2-pentanone, acetophenone and ethyl acetoacetate, sometimes in a yield suitable for preparative work. For each substrate, preferential reduction to the R-configurated alcohol was observed with one or more strains.
Similar content being viewed by others
References
Aragozzini F, Maconi E (1985) Riduzione di chetoni non ciclici con eumiceti. Ann Microbiol Enzimol (in press)
Bernardi R, Cardillo R, Ghiringhelli D (1984) Formation of the (R)- and (S)-enantiomers of ethyl 3-hydroxybutanoate and of 1-(1,3-dithian-2-yl)-2-hydroxypropane by microbial reduction. J Chem Soc, Chem Commun: 460–461
Brooks DW, Castro de Lee N, Peevey R (1984) Remote substituent effects in microbial reductions of 3-ketoglutarate and 3-ketoadipate esters. Tetrahedron Lett 25:4623–4626
Dale JA, Dull DL, Mosher HS (1969) α-Methoxy-α-trifluoromethylphenylacetic acid, a versatile reagent for the determination of enantiomeric composition of alcohols and amines. J Org Chem 34:2543–2549
Fischli A (1980) Chiral building blocks in enantiomer synthesis using enzymatic transformation. In: Eberson L, Seebach D, Vasella A, Fischli A (eds) Modern synthetic methods 1980. Otto Salle Verlag, Frankfurt am Main, p 269
Hirama M, Shimizu M, Iwashita M (1983) Enantiospecific syntheses of trifunctional (R)-3-hydroxy esters by baker's yeast reduction. J Chem Soc, Chem Commun: 599–600
Lanzilotta RP, Bradley DG, McDonald KM (1974) Microbial reduction of ketopantoyl lactone to pantoyl lactone. Appl Microbiol 27:130–134
MacLeod R, Prosser H, Fikentscher L, Lanyi J, Mosher HS (1964) Asymmetric reductions. XII. Stereoselective ketone reductions by fermenting yeast. Biochemistry 3:838–846
Nakamura K, Ushio K, Oka S, Ohno A (1984) Stereochemical control in yeast reduction. Tetrahedron Lett 25:3979–3982
Prelog V (1964) Specification of the stereospecificity of some oxido-reductase by diamond lattice sections. Pure Appl Chem 9:119–130
Seebach D, Züger MF, Giovannini F, Sonnleitner B, Fiechter A (1984) Preparative microbial reduction of β-oxoesters withThermoanaerobium brockii. Angew Chem Int Ed Engl 23:151–152
Sih CJ, Zhou B, Gopalan AS, Shieh W, Van Middlesworth F (1984) Strategies for controlling the stereochemical course of yeast reductions. In: Bartmann W, Trost BM (eds) Selectivity, a goal for synthetic efficiency. Workshop Conferences Hoechst, vol 14, Verlag Chemie. Berlin, pp 251–261
Velluz L, Valls J, Nominè G (1965) Fortschritte in der totalsynthese von der steroiden. Angew Chem 77:181–189
Wipf B, Kupfer B, Bertazzi R, Luenberger HG (1983) Production of (+)-(S)-ethyl 3-hydroxybutyrate and (-)-(R)-ethyl 3-hydroxybutyrate by microbial reduction of ethyl acetoacetate. Helv Chim Acta 66:485–488
Zhou B, Gopalan AS, Van Middlesworth F, Shieh W, Sih CJ (1983) Stereochemical control of yeast reductions. 1. Asymmetric synthesis of L-carnitine. J Am Chem Soc 105:5925–5926
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aragozzini, F., Maconi, E. & Craveri, R. Stereoselective reduction of non-cyclic carbonyl compounds by some eumycetes. Appl Microbiol Biotechnol 24, 175–177 (1986). https://doi.org/10.1007/BF00938791
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00938791