Abstract
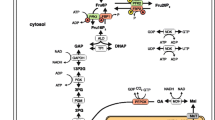
A mathematical model based on kinetic data taken from the literature is presented for the pentose phosphate pathway in fasted rat liver steady-state. Since the oxidative and non oxidative pentose phosphate pathway can act independently, the complete (oxidative + non oxidative) and the non oxidative pentose pathway were simulated.
Sensitivity analyses are reported which show that the fluxes are mainly regulated by D-glucose-6-phosphate dehydrogenase (for the oxidative pathway) and by transketolase (for the non oxidative pathway). The most influent metabolites were the group ATP, ADP, P1 and the group NADPH, NADP+ (for the non oxidative pathway).
Similar content being viewed by others
Abbreviations
- GK:
-
Glucokinase, (E.C. 2.7.1.2.)
- G6PDH:
-
D-glucose-6-phosphate dehydrogenase, (E.C. 1.1.1.49)
- PLase:
-
6-Phosphogluconelactonase, (E.C. 3.1.1.31.)
- PGIcDH:
-
6-Phosphogluconate dehydrogenase, (E.C. 1.1.1.44)
- RPI:
-
D-ribose-5-phosphate keto-isomerase, (E.C. 5.3.1.6)
- TK:
-
D-sedoheptulose-7-phosphate: D-glyceraldehyde-3-phosphate glycol-aldehyde transferase, (E.C. 2.2.1.1.)
- TA:
-
D-sedoheptulose-7-phosphate: D-glyceraldehyde-3-phosphate dihydroxyacetone transferase, (E.C. 2.2.1.2)
- EP:
-
D-ribulose-5-phosphate-3′-epimerase, (E.C. 5.1.3.1)
- PGI:
-
D-glucose-6-phosphate keto-isomerase, (E.C. 5.3.1.9)
- TPI:
-
D-glyceraldehyde-3-phosphate keto-isomerase, (E.C.5.3.1.1)
References
Kanji MI, Tows ML, Carper WR: A kinetic study of glucose-6-phosphate dehydrogenase. J Biol Chem 251: 2258–2262, 1976
Bauer HP, Srihari T, Jochims JT, Hofer HW: 6-Phosphogluconolactonase. Purification, properties and activities in various tissues. Eur J Biochem 133: 163–168, 1983
Schofield PJ, Sols A: Rat liver 6-phosphogluconolactonase: a low Km enzyme. Biochem Biophys Res Commun 71: 1313–1319, 1976
Villet RH, Dalziel K: Studies of 6-phosphogluconate dehydrogenase from sheep liver. I. Kinetics of the reductive carboxylation reaction. Eur J Biochem 27: 244–250, 1972
Toews ML, Kanji MI, Carper WR: 6-Phosphogluconate dehydrogenase. Purification and kinetics. J Biol Chem 251: 7127–7131, 1976
Topham CM, Dalziel K: The chemical mechanism of sheep liver 6-phosphogluconate dehydrogenase. A Schiff-base intermediate is not involved. Biochem J 234: 671–677, 1986
Gerber G, Preissler H, Heinrich R, Rapoport SM: Hexokinase of human erythrocytes. Purification, kinetic model, and its application to the conditions in the cell. Eur J Biochem 45: 39–52, 1974
Kiely ME, Stuart AL, Wood T: Partial purification and kinetic properties of ribose 5-phosphate ketol-isomerase and ribulose 5-phosphate 3-epimerase from various sources. Biochim Biophy Acta 293: 534–541, 1973
Dobrogosz WJ, Demoss RD: Studies on the regulation of ribosephosphateisomerase activity inPediococcus pentosaceus. Biochim Biophys Acta 77: 629–638, 1963
Domagk GF, Alexander WR: In: WA Wood (ed.). Methods in Enzymology vol. 41. Academic Press, New York, 1975, pp 424–426
Urivetzky M, Tsuboi KK: Enzymes of the human erythrocyte. V. Pentose phosphate isomerase, purification and properties. Arch Biochem Biophys 103: 1–8, 1963
McIntyre LM, Thorburn DR, Bubb WA, Kuchel PW: Comparison of computer simulations of the F-type and L-type non-oxidative hexose monophosphate shunts with31P-NMR experimental data from human erythrocytes. Eur J Biochem 180: 399–420, 1989
Wood T: The preparation of transketolase free from D-ribulose-5-phosphate 3-epimerase. Biochim Biophys Acta 659: 233–243, 1981
Canela EI: A program for deriving rate-equations using small computers. Int Biomed Compt 14: 43–52, 1983
Wood T: The Pentose Phosphate Pathway. Academic Press, Inc. London, 1985
Novello F, McLean P: The pentose phosphate pathway of glucose metabolism. Measurement of the non-oxidative reactions of the cycle. Biochem J 107: 775–791, 1968
Ljungdahl L, Wood HG, Racker E, Couri D: Formation of unequally labeled fructose 6-phosphate by an exchange reaction catalyzed by transaldolase. J Biol Chem 236: 1622–1630, 1961
Horecker BL, Smyrniotis PZ: Purification and properties of yeast transaldolase. J Biol Chem 212: 811–855, 1955
Kuhn E, Brand K: Purification and properties of transaldolase from bovine mammary gland. Biochemistry 11: 1767–1722, 1972
Hurwitz J, Horecker BL: The purification of phosphoketopentoepimerase fromLactobacillus pentosus and the preparation of xylulose 5-phosphate. J Biol Chem 223: 993–1008, 1956
Tsolas O, Horecker BL: In P. Boyer (ed.) The Enzymes ‘Transaldolase’, Vol. 7 1972
Wood T: Purification and properties of D-ribulose-5-phosphate 3-epimerase from calf liver. Biochim Biophys Acta 570: 352–362, 1979
Kauffman FC: Quantitative histochemistry of enzymes of the pentose phosphate pathway in the central nervous system of the rat. J Neurochem 19: 1–9, 1972
Kahana SE, Lowry OH, Schulz DW, Passoneau JV, Crawford EJ: The kinetics of phosphoglucoisomerase. J Biol Chem 235: 2178–2181, 1960
Lee EW, Barriso JA, Pepe M, Snyder R: Purification and properties of liver triose phosphate isomerase. Biochim Biophys Acta 242: 261–267, 1971
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sabate, L., Franco, R., Canela, E.I. et al. A model of the pentose phosphate pathway in rat liver cells. Mol Cell Biochem 142, 9–17 (1995). https://doi.org/10.1007/BF00928908
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00928908