Abstract
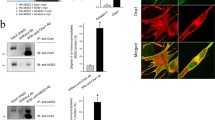
Phospholamban (PLB) is a regulator of the sarcoplasmic reticulum Ca2+-ATPase (SERCA2) expressed in cardiac, slow-twitch skeletal, and smooth muscles. Phospholamban is not expressed in the sarcoplasmic reticulum of fast-twitch skeletal muscle, but it can regulate the sarcoplasmic reticulum Ca2+-ATPase activity (SERCA1) expressed in this muscle,in vitro. To determine whether phospholamban can regulate SERCA1 activity in its native membrane environment, phospholamban was stably transfected into a cell line (C2C12) derived from murine fast-twitch skeletal muscle. Differentiation of C2C12 myoblasts to myotubes was associated with induction of SERCA1 expression, assessed by Western blotting analysis using Ca2+-ATPase isoform specific antibodies. The expressed phospholamban protein was localized in the microsomal fraction isolated from C2C12 myotubes. To determine the effect of phospholamban expression on SERCA1 activity, microsomes were isolated from transfected and nontransfected C2C12 cell myotubes, and the initial rates of45Ca2+-uptake were determined over a wide range of Ca2+ concentrations (0.1–10 μM). Expression of phospholamban was associated with inhibition of the initial rates of Ca2+-uptake at low [Ca2+] and this resulted in a decrease in the affinity of SERCA1 for Ca2+ (0.27±0.02 μM in nontransfected vs. 0.41±0.03 μM in PLB transfected C2C12 cells). These findings indicate that phospholamban expression in C2C12 cells is associated within inhibition of the endogenous SERCA1 activity and provide evidence that phospholamban is capable of regulating this Ca2+-ATPase isoform in its native membrane environment.
Similar content being viewed by others
References
MacLennan DH, Brandl CJ, Korczak B, Green NM: Amino acid sequence of a Ca2+ and Mg2+-dependent ATPase from rabbit muscle SR, deduced from its complementary DNA sequence. Nature 316: 696–700, 1985
Brandl CJ, Green NM, Korczak B, MacLennan DH: Two Ca2+-ATPase genes: Homologies and mechanistic implications of deduced amino acid sequences. Cell 44: 597–607, 1986
Brandl CJ, deLeon S, Martin DR, MacLennan DH: Adult forms of the Ca2+-ATPase of SR: Expression in developing skeletal muscle. J Biol Chem 262: 3768–3774, 1987
Lytton J, MacLennan DH: Molecular cloning of cDNAs from human kidney coding for two alternatively spliced products of the cardiac Ca2+-ATPase gene. J Biol Chem 263: 15024–15031, 1988
Burk SE, Lytton J, MacLennan DH, Shull GE: cDNA cloning, functional expression, and mRNA tissue distribution of a third organellar Ca2+-pump. J Biol Chem 264: 18561–18568, 1989
Arai M, Otsu K, MacLennan DH, Periasamy M: Regulation of SR gene expression during cardiac and skeletal muscle development. Am. J. Physiol. 262: C614–C620, 1992
Gunteski-Hamblin A-M, Greeb J, Shull G: A novel Ca2+-pump expressed in brain, kidney, and stomach is encoded by an alternative transcript of the slow-twitch muscle sarcoplasmic reticulum Ca-ATPase gene. J Biol Chem 263: 15032–15040, 1988
Ferguson DG, Franzini-Armstrong C: The Ca2+-ATPase content of slow and fast twitch fibers of guinea pig. Muscle and Nerve 11: 561–570, 1988
Kim HW, Steenaart NAE, Ferguson DG, Kranias EG: Functional reconstitution of the cardiac sarcoplasmic reticulum Ca2+-ATPase with phospholamban in phospholipid vesicles. J Biol Chem 265: 1702–1709, 1990
Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH: Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem 267: 14483–14489, 1992
Toyofuku T, Kurzydlowski K, Lytton J, MacLennan DH: The nucleotide binding/hinge domain plays a crucial role in determining isoform-specific Ca2+-dependence of organellar Ca2+-ATPases. J. Biol. Chem. 267: 14490–14496, 1992
Cantilina, T., Sagara, Y., Inesi, G., Jones, LR.: Comparative studies of cardiac and skeletal SR ATPases: Effect of a phospholamban antibody on enzyme activation by Ca2+. J Biol Chem 268: 17018–17025, 1993
Tada M, Kirchberger MA, Repke DI, Katz AM: The stimulation of calcium transport in cardiac SR by adenosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem 249: 6174–6180, 1974
Wang T, Grassi de Gende AO, Schwartz A: Kinetic properties of calcium adenosine triphosphatase of SR isolated from cat skeletal muscle: A comparison of caudofemoralis (fast), tibialis (mixed), and soleus (slow). J Biol Chem 254: 10675–10678, 1979
Fujii J, Ueno A, Kitano K, Tanaka S, Kadoma M, Tada M: Complete complementary DNA-derived amino acid sequence of canine cardiac phospholamban. J Clin Invest 79: 301–304, 1987
Jorgensen AO, Jones LR: Localization of PLB in slow but not fast canine skeletal muscle fibers: An immunocytochemical and biochemical study. J Biol Chem 261: 3775–3781, 1986
Ferguson DG, Young EF, Raeymaekers L, Kranias EG: Localization of phospholamban in smooth muscle using immunogold electron microscopy. J Cell Biol 107: 555–562, 1988
Tada M, Ohmori F, Yamada M, Abe H: Mechanism of the stimulation of Ca2+-dependent ATPase of cardiac SR by adenosine 3′:5′-monophosphate dependent protein kinase. J Biol Chem 254: 319–326, 1979
Kranias EG, Mandel F, Wang T, Schwartz A: Mechanism of the stimulation of Ca2+-dependent ATPase of cardiac sarcoplasmic reticulum by adenosine 3′:5′-monophosphate-dependent protein kinase. Biochemistry 19: 5434–5439, 1980
Tada M, Yamada M, Ohmori F, Kuzuya T, Inui M, Abe H: Transient state kinetic studies of Ca2+-dependent ATPase and calcium transport by cardiac SR. Effect of cyclic AMP-dependent protein kinasecatalyzed phosphorylation of phospholamban. J Biol Chem 255: 1985–1992, 1980
James P, Inui M, Tada M, Chiesi M, Carafoli E: Nature and site of phospholamban regulation of the Ca2+ pump of sarcoplasmic reticulum. Nature 342: 90–92, 1989
Kirchberger MA, Tada M, Katz AM: Adenosine 3′:5′-monophosphate dependent protein kinase-catalyzed phosphorylation reaction and its relationship to calcium transport in cardiac sarcoplasmic reticulum. J Biol Chem 249: 6166–6173, 1974
LaRaia PJ, Morkin F: Adenosine 3′,5′-monophosphate-dependent membrane phosphorylation, a possible mechanism for the control of microsomal calcium transport in heart muscle. Circ Res 35: 298–305, 1974
Tada M, Kirchberger MA, Katz AM: Phosphorylation of a 22,000-dalton component of the cardiac SR by adenosine 3′:5′-monophosphate dependent protein kinase. J Biol Chem 250: 2640–2647, 1975
LePeuch AJ, Haiech J, Demaille JG: Concerted regulation of cardiac sarcoplasmic reticulum calcium transport by cyclic adenosine monophosphate dependent and calcium-calmodulin-dependent phosphorylations. Biochemistry 18: 5150–5157, 1979
Bilezikjian LM, Kranias EG, Potter JD, Schwartz A: Studies on phosphorylation of canine cardiac SR by calmodulin-dependent protein kinase. Circ Res 49: 1356–1362, 1981
Kirchberger MA, Antonetz T: Calmodulin-mediated regulation of Ca2+-transport and (Ca2+ and Mg2+)-activated activity in isolated cardiac SR. J Biol Chem 257: 5685–5691, 1982
Davis BA, Schwartz A, Samaha FJ, Kranias EG: Regulation of cardiac sarcoplasmic reticulum calcium transport by calcium-calmodulin dependent phosphorylation. J Biol Chem 258: 13587–13591, 1983
Movsesian MA, Nishikawa M, Adelstein RS: Phosphorylation of phospholamban by calcium-activated, phospholipid-dependent protein kinase. J Biol Chem 259: 8029–8032, 1984
Kimura Y, Inui M, Kadoma M, Kijima Y, Sasaki T, Tada M: Effects of monoclonal antibody against phospholamban on calcium pump ATPase of cardiac sarcoplasmic reticulum. J Mol Cell Card 23: 1223–1230, 1991
Morris GL, Cheng H, Colyer J, Wang JH: Phospholamban regulation of cardiac sarcoplasmic reticulum (Ca2+−Mg2+)-ATPase: Mechanism of regulation and site of monoclonal antibody interaction. J Biol Chem 266: 11270–11275, 1991
Sham J, Jones LR, Morad M: Phospholamban mediates the β-adrenergic-enhanced Ca2+ in mammalian ventricular myocytes. Am J Physiol 261: H1344–H1349, 1991
Briggs FN, Lee KF, Wechsler AW, Jones LR: Phospholamban expressed in slow-twitch and chronically stimulated fast-twitch muscles minimally affects calcium affinity of sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem 267: 26056–26061, 1992
Szymanska G, Kim HW, Cuppoletti J, Kranias EG: Regulation of the skeletal sarcoplasmic reticulum Ca2+-pump by phospholamban in reconstituted phospholipid vesicles. Membrane Biochem 9: 191–202, 1992
Fujii J, Maruyama K, Tada M, MacLennan DH: Co-expression of slow-twitch/cardiac muscle Ca2+-ATPase (SERCA2) and phospholamban. FEBS Lett 273: 232–234, 1990
Toyofuku T, Kurzydlowski K, Tada M, MacLennan DH: Identification of regions in the Ca2+-ATPase of sarcoplasmic reticulum that affect functional association with phospholamban. J Biol Chem 268: 2809–2815, 1993
Toyofuku T, Kurzydlowski K, Tada M, MacLennan DH: Amino acids Glu2 to Ile18 in the cytoplasmic domain of phospholamban are essential for functional association with the Ca2+-ATPase of sarcoplasmic reticulum. J Biol Chem 269: 3088–3094, 1994
Yaffe D, Saxel O: Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270: 725–727, 1977
Blau HM, Chiu C-P, Webster C: Cytoplasmic activation of human genes in stable heterocaryons. Cell 32: 1171–1180, 1983
Miyazaki J, Takaki S, Araki K, Tashiro F, Tominaga A, Takatsu K, Yamamura K: Expression vector system on the chicken β-actin promoter directs efficient production of interleukin-5. Gene 79: 269–277, 1989
Ganim JR, Luo W, Ponniah S, Grupp I, Kim HW, Ferguson DG, Kadambi V, Neumann JC, Doetschman T, Kranias EG: Mouse phospholamban gene expression during developmentin vivo andin vitro. Circ Res 71: 1021–1030, 1992
Chen C, Okayama H: High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7: 2745–2752, 1987
Stambrook J, Fritsch EF, Maniatis T: Gel electrophoresis of DNA. In: N. Ford, C. Nolan, M. Ferguson (eds). Molecular Cloning: A laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, 1989, pp 6.3–6.62
Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinum thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159, 1987
Maruyama K, MacLennan DH: Mutation of aspartic acid-351, lysine-352 and lysine-515 alters the Ca2+ transport activity of the Ca2+-ATPase expressed in COS-1 cells. Proc Natl Acad Sci USA 85: 3314–3318, 1989
Martonosi A, Feretos R: Sarcoplasmic reticulum: I. The uptake of Ca2+ by sarcoplasmic reticulum fragments. J Biol Chem 239: 648–658, 1964
Robertson S, Potter JD: The regulation of free Ca2+ ion concentration by metal chelators. In: A. Schwartz (ed.). Methods in Pharmacology, Plenum Press, NY, 1984, pp 63–75
Jorgenson AO, Arnold W, Pepper DR, Kahl SD, Mandel F, Campbell KP: A monoclonal antibody to the Ca2+-ATPase of cardiac sarcoplasmic reticulum cross-reacts with slow type I but not fast type II canine skeletal muscle fibers: An immunocytochemical and immunochemical study. Cell Motility and the Cytoskeleton 9: 164–174, 1988
Zarain-Herzberg A, MacLennan DH, Periasamy M: Characterization of rabbit cardiac sarco(endo)plasmic reticulum Ca2+-ATPase gene. J Biol Chem 265: 4670–4677, 1990
Higham SC, Melikian J, Karin NJ, Ismail-Beigi F, Pressley TA: Na,K-ATPase expression in C2C12 cells during myogenesis: Minimal contribution of α2 isoform to Na,K transport. J Membrane Biol 131: 129–136, 1993
DeSmedt HD, Eggermont JA, Wuytack F, Parys JB, VanDenBosch L, Missiaen L, Verbist J, Casteels R: Isoform switching of the sarco(endo)plasmic reticulum Ca2+ pump during differentiation of BC3H1 myoblasts. J Biol Chem 266: 7092–7095, 1991
Suzuki T, Lui P, Wang JH: The use of monoclonal antibodies for species and tissue distribution of phospholamban. Cell Calcium 7: 41–47, 1986
Huang MT, Gorman, CM: The simian virus 40 small-t intron, present in many common expression vectors, leads to aberrant splicing. Mol Cell Biol 10: 1805–1810, 1990
Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG: Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of β-agonist stimulation. Circ Res 75: 401–409, 1994
Suzuki T, Lui P, Wang JH: The phosphorylation of purified phospholamban by cyclic AMP-dependent protein kinase is stimulated by phosphatidylinoiitol. J Biol Chem 262: 3880–3885, 1987
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Harrer, J.M., Ponniah, S., Ferguson, D.G. et al. Expression of phospholamban in C2C12 cells and regulation of endogenous SERCA1 activity. Mol Cell Biochem 146, 13–21 (1995). https://doi.org/10.1007/BF00926876
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00926876