Abstract
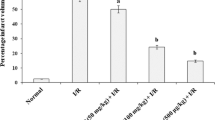
The aim of this study was to test the effect of vitamins A and E in reducing oxyradical effects and myocardial damage after ischemia — reperfusion in the rabbit heart. Oxyradical effects were indirectly assessed by hydroperoxide initiated chemiluminescence and myocardial damage was evaluated by qualitative and quantitative electron microscopy. Left anterior coronary artery was ligated in control and vitamin-treated rabbits for 30 min and then reperfused for 10 min. Rabbits were pretreated with 150 mg vitamin E and 60000 IU vitamin A 24 h before surgery. After 10 min of reperfusion full-thickness needle samples were obtained from five different myocardial areas (three ventricular and two septal areas) and used for the determination of hydroperoxide-initiated chemiluminescence and ultrastructural damage. In the control group, hydroperoxide-initiated chemiluminescence was 18400±500 cpm/mg protein for the non-ischemic and non-reperfused ventricular areas, and 40500±1800 cpm/mg protein for ischemic-reperfused ventricular areas. In the vitamin-treated group, hydroperoxide-initiated chemiluminescence was decreased by 8% in the non ischemic and non reperfused ventricular areas and by 51–75% in the ventricular ischemic and reperfused areas. The two septal areas in the control group gave chemiluminescences of 6800±1200 cpm/mg protein (non ischemic-non reperfused) and 17000±2000 cpm/mg protein (ischemia-reperfusion). In the vitamin-treated group, chemiluminescence decreased by 4 and 58%, respectively.
The ischemia-reperfused areas showed extensive edema, margination of nuclear chromatin and swollen mitochondria with disrupted cristae including rupture of the inner and outer mitochondrial membranes. Assessment of mitochondrial damage in electron micrographs by stereological counting and grading indicated 77% of damaged mitochondria. These hearts displayed the early sings of irreversible damage and infarction. Rabbits pretreated with vitamins A and E showed a 18% of damaged mitochondria in the same areas (p<0.001) and relative preservation of myocyte subcellular structures.
The results indicated that vitamins A and E reduce hydroperoxide-initiated chemiluminescence and myocardial cell damage during ischemia-reperfusion in the rabbit.
Similar content being viewed by others
References
Jennings RB, Sommers HM, Symth GA, et al.: Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol 70: 82–93, 1960
Opie L: Metabolism-emergence, decline and resurgence. Part II. Cardiovasc Res 26: 817–830, 1992
Guarnieri C, Flamigni F, Caldarera CM: Role of oxygen in the cellular damage induced by reoxygenation of hypoxic heart. J Mol Cell Cardiol 12: 797–808, 1988
Ferreira R, Llesuy S, Milei J, et al.: Assessment of myocardial oxidative stress in patients after myocardial revascularization. Am Heart J 115: 307–312, 1988
Stewart JR, Blackwell MS, Crute SL, et al.: Inhibition of surgically induced ischemia reperfusion injury by oxygen free radical scavengers. J Thorac Surg 86: 262–272, 1983
Menasche P, Grousset C, Grauduel Y, et al.: A comparative study of free radical scavengers in cardioplegic solution. Improved protection with peroxidase. J Thorac Cardiovasc Surg 92: 264–271, 1986
Bernier M, Hearse DJ, Manning AS: Reperfusion-induced arrhythmias and oxygen derived free radicals. Studies with antifree radical interventions and a free-radical generating system in the isolated perfused rat heart. Circ Res 58: 331–340, 1986
Hearse DJ, Tosaki A: Free radicals and reperfusion-induced arrhythmias: protection by the spin trap agent PBN in rat heart. Circ Res 60: 375–383, 1987
Naslund U, Haggmark S, Johansson G, et al.: Superoxide dismutase and catalase reduce infarct size in a porcine myocardial occlusion reperfusion model. J Mol Cell Cardiol 18: 1077–1084, 1986
Menasche P, Piwnica A: Free radicals and myocardial protection: A surgical viewpoint. Ann Thorac Surg 47: 939–945, 1989
Ferreira R, Burgos M, Llesuy S, et al.: Reduction of reperfusion injury with mannitol cardioplegia. Ann Thorac Surg 48: 77–84, 1989
Ferreira R, Burgos M, Milei J, et al.: Effect of supplementing cardioplegic solution with deferoxamine on reperfused human myocardium. J Thorac Cardiovasc Surg 100: 708–714, 1990
Milei J, Ferreira R, Llesuy S, et al.: Reduction of reperfusion injury with preoperative rapid intravenous infusion of taurine during myocardial revascularization. Am Heart J 123: 339–345, 1992
Ferreira R, Milei J, Llesuy S, et al.: Antioxidant action of vitamins A and E in patients submitted to coronary artery bypass surgery. Vasc Surgery 25: 191–195, 1991
González Flecha B, Llesuy S, Boveris A: Hydroperoxide initiated chemiluminescence: An assay for oxidative stress in biopsies of heart and liver. Free Rad Biol & Med 10: 93–100, 1991
Boveris A, Fraga CG, Varsavsky A et al.: Increased chemiluminescence and superoxide production in the liver of chronically ethanol-treated rats. Arch Biochem Biophys 227: 534–541, 1983
Fraga CG, Llesuy S, Boveris A: Increased carbon tetrachloridestimulated chemiluminescence in thein situ liver of barbital treated mice. Acta Physiol Pharmacol Latinoam 34: 143–150, 1984
Boveris A, Llesuy S, Fraga CG: Increased liver chemiluminescence in tumor-bearing mice. J Free Rad Biol & Med 1: 131–138, 1985
Milei J, Boveris A, Llesuy S, et al.: Amelioration of Adriamycin-induced cardiotoxicity in rabbits by prenylamine and vitamins A and E. Am Heart J 111: 95–102, 1986
Llesuy S, Milei J, Molina H, et al.: Comparison of lipid peroxidation and myocardial damage induced by adriamycin and 4-epiadriamycin in mice. Tumori 71: 241–248, 1985
Van Vleet JF, Greenwood L, Ferrans VJ et al.: Effec of selenium and vitamin E on adriamycin-induced cardiomyopathy in rabbits. Am J Vet Res 39: 998–1020, 1978
Breed JGS, Zimmerman ANB, Dormans JAMA, et al.: Failure of the antioxidant vitamin E to protect against Adriamycin-induced cardiotoxicity in the rabbit. Cancer Res 40: 2033–2040, 1980
Llesuy S, González Flecha B, Milei J, et al.: Myocardial damage induced by doxorubicins: hydroperoxide — initiated chemiluminescence and morphology. Free Rad Biol and Med 8: 259–264, 1990
Lowry OH, Rosengrough AL, Farr AL, et al.: Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–269, 1951
Kloner RA, Fishbein MC, Braunwald E, et al.: Effect of propanolol on mitochondrial morphology during acute myocardial ischemia. Am J Cardiol 41: 881–886, 1978
Thompson JA, Hess ML: The oxygen free radical system: a fundamental mechanism in the production of myocardial necrosis. Prog Cardiovasc Dis 28: 449–462, 1986
Hess ML, Manson NH, Okabe E: Involvement of free radicals in the pathogenesis of ischemic heart disease. Can J Physiol Pharmacol 60: 1382–1389, 1982
Freeman BA, Crapo JD: Biology of disease. Free radicals and tissue injury. Lab Invest 47: 412–424, 1982
Fehder J, Csomos G, Vereckei A: The chemistry of free radical reactions in medicine, Springer-Verlag, Berlin: 1987, p 2–10
White BC, Krause GS, Aust SD, et al.: Postischemic tissue injury by iron-mediated free radical lipid peroxidation. Ann Emerg Med. 136: 805–809, 1985
Foote CS: Photosensitized oxidation and singlet oxygen: consequences in biological systems. In: W.A. Pryor (ed.). Free Radicals in Biology. Academic Press, New York, 1976, vol 2, pp 85–134
Milei J, Llesuy S: Reduction in the toxicity of doxorubicin (Adriamycin) in the heart by Vitamin E. In: L. Packer and J. Fuchs (eds). Vitamin E in Health and Disease. Academic Press, Ny, 1993, pp 417–431
Mickle Dag, Li RK, Weisel RD, Bionbaum PL, Wu TW, Jackowski G, Madonik MM, Burton GW, Ingold KU: Myocardial salvage with trolox and ascorbic acid for an acute evolving infarction. Ann Thorac Surg 47: 553–7, 1989
Massey KD, Burton KP: α-tocopherol attenuates myocardial membrane-related alterations resulting from ischemia and reperfusion. Am J Physiol 256: H1192-H1199, 1989
Massey KD, Burton KP: Free radical damage in neonatal rat cardiac myocyte cultives: Effects of α tocopherol, Trolox and phytol. Free Rad Biol and Med 8: 449–458, 1990
Oliveria M, Weglicki W, Nason A, et al.: Distribution of tocopherol in beef heart mitochondria. Biochem Biophys Acta 180: 98–113, 1969
Witting L: Vitamin E and lipid antioxidants in free radical initiated reactions. In: W.A. Pryor (ed.). Free Radicals in Biology. Academic Press, New York, 1980, vol IV, pp 295–320
Guarnieri C, Flamigni F, Rossoni-Caldarera C: Myocardial mitochondrial functions in tocopherol-deficient and refed rabbits. Adv Myocardiol 3: 621–626, 1982
Flamigni F, Guarnieri C, Toni R, et al.: Effect of oxygen radicals on heart mitochondrial function in tocopherol deficient rabbits. Internat J Vit Nutr Res 52: 402–406, 1982
Barascchi R, Pelosi G, Maffei G, et al.: Myocardial vitamin E is consumed during cardiopulmonary bypass: indirect evidence of free radical generation in human ischemic heart. Int J Cardiol 37: 339–343, 1992
Flitter WD, Coghlan JG, Clutton SM, et al.: The role of free radicals injury. Free Rad Res Comm 16: 20, 1992
Chambers DE, Parks DA, Patterson G, et al.: Xanthine oxidase as a source of free radical damage in myocardial ischemia. J Mol Cell Cardiol 17: 145–52, 1985
Eddy LJ, Stewart JR, Jones HP, et al.: The free radical-producing enzyme, xanthine oxidase, is undetectable in human hearts. Am J Physiol 253: H709–711, 1987
Downey J, Miura T, Eddy L, et al.: Xanthine oxidase is not a source of free radicals in the ischemic rabbit heart. J Mol Cell Cardiol 19: 1053–1060, 1987
Boveris A, Chance B: The mitochondrial generation of hydrogen peroxide: General properties and effect of hyperbaric oxygen. Biochem J 134: 107–116, 1973
McCord JM: Free radicals and myocardial ischemia: overview and outlook. J Free Radical Biol Med 4: 9–14, 1988
González Flecha B, Cutrín JC, Boveris A: Time course and mechanism of oxidative stress and tissue damage in rat liver subjected toin vivo ischemia-reperfusion. J Clin Invest 91: 456–464, 1993
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Llesuy, S., Milei, J., Picone, V. et al. Effect of vitamins A and E on ischemia-reperfusion damage in rabbit heart. Mol Cell Biochem 145, 45–51 (1995). https://doi.org/10.1007/BF00925712
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00925712