Abstract
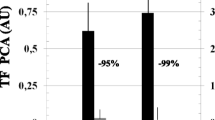
Human serurn was found to contain an inhibitor of constitutive interleukin 1 (IL-1) production by human umbilical vein endothelial cells (ECs). Purification of the serum activity by anion exchange chromatography, molecular sieve HPLC, and hydroxyl apatite chromntography yielded material 82% pure with a molecular weight of 17 kDa by SDS-PAGE. Amino acid sequencing revealed the purified inhibitor to be transthyretin (TTR). a liver-derived protein. There was a 42.6% reduction in the production of spontaneous IL-1 activity in EC supernatants after coculture with 10μg/ml TTR. TTR was subsequently found by ELISA to inhibit LPS-stimulated IL-1 production by cells of the human monocytic leukemia line THP-1 by 47.1 ± 9.4%, whereas a less striking but still significant inhibition of monocyte-derived IL-1β production was also observed. Inhibition of IL-1 secretion correlated with increased IL-1 mRNA synthesis in both THP-1 cells and monocytes. Furthermore, TTR was associated with increased intracellulaf concentrations of IL-1β. These data suggest that TTR functions by inhibiting processing of newly synthesized peptide for secretion. This novel inhibitory effect of TTR on the production of IL-1 activity suggests a previously unrecognized endogenous antiinflammatory mediator.
Similar content being viewed by others
References
Rosenwasser, L. J. 1984. Interleukin 1: An overview.Proc IUPHAR 9th Int. Cong. Pharmacol. 3:301–306.
Dinarello, C. A. 1984. Interleukin-1.Rev. Infect. Dis. 6:51–95.
Dinarello, C. A., J. G. Cannon, J. W. Mier, H. A. Bernheim, G. Lopreste, D. L. Lynn, R. N. Love, A. C. Webb, P. E. Auron, R. C. Reuben, A. Rich, S. M. Wolff, andS. D. Putney. 1986. Multiple biological activities of human recombinant interleukin-1.J. Clin. Invest. 77:1734–1739.
Rosenwasser, L. J., andC. A. Dinarella. 1981. Ability of human leukocytic pyrogen to enhance phytohemagglutinin induced murine thymocyte proliferation.J. Cell. Immunol. 63:134–142.
Auron, P. E., A. C. Webb, L. J. Rosenwasser, S. F. Mucci, A. Rich, S. M. Wolff, andC. A. Dinarello. 1984. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA.Proc. Natl. Acad. Sci. U.S.A. 81:7907–7911.
Miossec, P., D. Cavender, andM. Ziff. 1986. Production of interleukin-1 by human endothelial cells.J. Immunol. 136:2486–2491.
Marucha, P. T., R. A. Zeff, andD. L. Kreutzer. 1990. Cytokine regulation of IL-1β gene expression in the human polymorphonuclear leukocyte.J. Immunol. 145:2932–2937.
Sauder, D. N., C. S. Carter, S. I. Katz, andJ. J. Openheim. 1982. Epidermal cell production of thymocyte activating factor (ETAF).J. Invest. Dermatol. 79:34–39.
Larrick, J. W. 1989. Native interleukin-1 inhibitors.Immunol. Today 10:61–66.
Seckinoer, P., J. W. Lowenthal, K. Williamson, J.-M. Dayer, andH. R. MacDonald. 1987. A urine inhibitor of interleukin 1 activity that blocks ligand binding.J. Immunol. 139:1546–1549.
Hannum, C. H., C. J. Wilcox, W. P. Arend, F. G. Joslin, D. J. Dripps, P. L. Heimdal, L. G. Armes, A. Sommer, S. P. Eisenberg, andR. C. Thompson. 1990. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor.Nature 343:336–340.
Gehrke, L., S. A. Jobling, L. S. K. Pak, B. McDonald, L. J. Rosenwasser, andP. E. Auron. 1990. A point mutation uncouples human interleukin-1β biological activity and receptor binding.J. Biol. Chem. 265:5922–5925.
King, M., S. M. Wolff, andL. J. Rosenwasser. 1987. Vasculitic sera lack an inhibitor of endothelial cell IL-1 production.Clin. Res. 35:608A.
Libby, P., J. M. Ordovas, K. R. Auger, A. H. Robbins, L. K. Birinyi, andC. A. Dinarello. 1986. Endotoxin and tumor necrosis factor induce interleukin 1 gene expression in adult human vascular endothelial cells.Am. J. Pathol. 124:179–185.
Rosenwasser, L. J., P. E. Auron, L. Gehrke, B. Clark, B. McDonald, B. Bradley, E. Epstein, K. Collins, andA. C. Webb. 1987. IL-1: Review of structure and function, definition of an active site for T cells and production by cultured vascular endothelium.In Biologically Based Immunomodulators in Rheumatic Diseases. S. Pincus, D. Pisetsky, and L. J. Rosenwasser, editors. Elsevier Publishers, New York. 247–256.
Maciag, T., J. Cerundolo, S. Ilsley, P. R. Kelley, andR. Forand. 1979. An endothelial cell growth factor from bovine hypothalamus: Identification and partial characterization.Proc. Natl. Acad. Sci. U.S.A. 76:5674–5678.
Jaffe, E. A., E. R. Nachman, C. E. Becker, andC. Minick. 1973. Culture of human endothelial cells derived from umbilical veins: Identification by morphologic and immunologie criteria.J. Clin. Invest. 52:2745–2756.
Rosenwasser, L. J., C. A. Dinarello. 1981. Ability of human leukocytic pyrogen to enhance phytohemaglutinin-induced murine thymocyte from proliferation.J. Cell. Immunol. 63:134.
Kaye, J., S. Porcelli, J. Tite, B. Jones, andC. A. Janeway. 1983. Both a monoclonal antibody and antisera specific for determinants unique to individual cloned helper T cell lines can substitute for antigen and antigen-presenting cells in the activation of T cells.J. Exp. Med. 158:836–856.
Jobling, S. A., P. E. Auron, G. Gurka, A. C. Webe, B. McDonald, L. J. Rosenwasser, andL. Gehrke. 1988. Biological activity and receptor binding of human prointerleukin 1β and subpeptides.J. Biol. Chem. 263:16372–16378.
Jobling, S. A., andL. Gehrke. 1987. Enhanced translation of chimaeric messenger RNAs containing a plant viral untranslated leader sequence.Nature 325:622–625.
Pearson, W. R., andD. J. Lipman. 1988. Improved tools for biological sequence comparison.Proc. Natl. Acad. Sci. U.S.A. 85:2444–2448.
Sasaki, H., H. Yoshicka, N. Takagi, andY. Sakaki. 1985. Structure of the chromosomal gene for human serum prealbumin.Gene 37:191–197.
Zucali, J. R., H. E. Broxmeyer, D. Levy, andC. Morse. 1989. Lactoferrin decreases monocyte-induced fibroblast production of myeloid colony-stimulating activity by suppressing monocyte release of interkukin-1.Blood 74:1531–1536.
Dinarello, C. A., L. J. Rosenwasser, andS. M. Wolff. 1981. Demonstration of a circulating suppressor factor of thymocyte proliferation during endotoxin fever in humans.J. Immunol. 27:2517–2521.
Liao, Z., R. S. Grimshaw, andD. L. Rosenstreich. 1984. Identification of a specific interleukin 1 inhibitor in the urine of febrile patients.J. Exp. Med. 159:126–136.
Rosenstreich, D. L., J. H. Tu, P. R. Kinkade, I. Maurer-Fogy, J. Kahn, R. W. Barton, andP. R. Farina. 1988. A human urine derived interleukin-1 inhibitor: Homology with deoxyribonuclease I.J. Exp. Med. 168:1767–1779.
Ferguson, R. N., H. Edelhoch, H. A. Saroff, andJ. Robbins. 1975. Negative cooperativity in the binding of thyroxine to human serum prealbumin.Biochemistry 14:282–289.
Soprano, D. R., K. J. Soprano, M. L. Wyatt, andD. S. Goodman. 1988. Induction of the expression of retinol-binding protein and transthyretin in F9 embryonal carcinoma cells differentiated to embryoid bodies.J. Biol. Chem. 263:17897–17900.
Woeber, K. A., andS. H. Ingbar. 1968. The contribution of throxine-binding prealbumin to the binding of thyroxine in human serum, as assessed by immunoadsorption.J. Clin. Invest. 47:1710–1721.
Gorevic, P. D., F. C. Prelli, J. Wright, M. Pras, andB. Frangione. 1989. Systemic senile amyloidosis: Identification of a new prealbumin (transthyretin) variant in cardiac tissue: Immunologic and biochemical similarity to one form of familial amyloidotic polyneuropathy.J. Clin. Invest. 83:836–843.
Dickson, P. W., A. R. Aldred, P. D. Marley, D. Bannister, andG. Schreiber 1986. Rat choroid plexus specializes in the synthesis and the secretion of transthyretin (prealbumin).J. Biol. Chem. 261:3475–3478.
Douville, P., J. Talbot, R. Lapointe, andL. Belager. 1982. Potential usefulness of serum prealbumin in total parenteral nutrition.Clin. Chem. 28:1706–1707.
Connors, L. H., M. A. Gertz, M. Skinner, andA. S. Cohen. 1984. Nephelometric measurement of human serum prealbumin and correlation with acute phase proteins CRP and SAA: Results in familial amyloid polyneuropathy.J. Lab. Clin. Med. 104:538–545.
Darlington, G. J., D. R. Wilson, andL. B. Lachman. 1986. Monocyte conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro.J. Cell. Biol. 103:787–793.
Gauldie, J., C. Richards, D. Harnish, P. Lansdorp, andH. Bauman. 1987. Interferon β2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte stimulatory factor and regulates the major acute phase protein response in liver cells.Proc. Natl. Acad. Sci. U.S.A. 84:7251–7256.
Gauldie, J., D. N. Sauder, K. P. W. J. McAdam, andC. A. Dinarello. 1987. Purified interleukin-1 (IL-1) from human monocytes stimulates acute phase protein synthesis by rodent hepatocytes in vitro.Immunology 60:203–207.
Billiau, A. 1986. BSF-2 is not just a differentiation factor.Nature 324:415.
Van Damme, J., S. Cayphas, G. Opdenakker, A. Billiau, andJ. Van Snick. 1987. Interleukin 1 and poly (rI)·poly (rC) induces production of a hybridoma growth factor by human fibroblasts.Eur. J. Immunol. 17:1–7.
Black, R. A., S. Kronheim, andP. Sleath. 1989. Activations of interleukin-1 beta by a co-induced protease.Fed. Eur. Biol. Soc. Lett. 247:386–390.
Kostura, M. J., J. Tocci, G. Limjuco, J. Chin, P. Cameron, A. Hillman, N. Chartrain, andJ. Schmidt. 1989. Identification of a monocyte specific preinterleukin-1-beta convertase activity.Proc. Natl. Acad. Sci. U.S.A. 86:5227–5231.
Black, R., S. Kronheim, J. Merriam, C. J. March, andT. Hopp. 1989. A preaspartate-specific protease from human leukocytes that cleaves prointerleukin-1-beta.J. Biol. Chem. 264:5323–5326.
Young, P., D. Hazuda, andP. Simon. 1988. Human IL-1β is not secreted from hamster fibroblasts when expressed constitutively from transfected cDNA.J. Cell. Biol. 107:447–456.
Fuhlbrioge, R., S. Fine, E. Unanue, andD. Chaplin. 1988. Expression of membrane interleukin-1 by fibroblasts transfected with murine prointerleukin-1-alpha cDNA.Proc. Natl. Acad. Sci. U.S.A. 85:5649–5653.
Mizutani, H., R. Black, andT. S. Kupper. 1991. Human keratinocytes produce but do not process pro-interleukin-1 (IL-1) beta: Different strategies of IL-1 production and processing in monocytes and keratinocytes.J. Clin. Invest. 87:1066–1071.
Wewers, M. D., andD. J. Herzyk. 1989. Alveolar macrophages differ from blood monocytes in human IL-1β release: Quantitation by enzyme-linked immunoassay.J. Immunol. 143:1635–1641.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Borish, L., King, M.S., Mascali, J.J. et al. Transthyretin is an inhibitor of monocyte and endothelial cell interleukin-1 production. Inflammation 16, 471–484 (1992). https://doi.org/10.1007/BF00918973
Issue Date:
DOI: https://doi.org/10.1007/BF00918973