Abstract
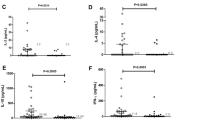
Patients with schistosomiasis of the urinary bladder (SB) and schistosomiasis with carcinoma of the urinary bladder (SCB) had significantly increased percentage of IL-2R-, HLA-DR-, and transferrin receptor (T9)-bearing T cells in circulation. The percentage of these activated T cells decreased byin vitro culture with PHA but increased bySchistosoma haematobium soluble egg antigen. These patients with SB and SCB had a PHA-specific defect in IL-2 production. A functional defect with induction of IL-2R-positive suppressor monocytes appears to correlate with the defective PHA-induced IL-2 production by CD4+ T cells and monocytes. Disordered regulation of PHA-induced IL-2 production by the CD4+ T cells and monocytes may be the key feature in the highly depressed cell-mediated immune response in schistosomiasis. However, immune activation and significantly elevated IL-2 production in response to disease-specificS. haematobium soluble egg antigen may be related with the pathogenesis of SB and SCB. Thus,S. haematobium-specific CD4+ T cells are present in schistosomiasis, and their function is determined by adequate release of IL-2-and/or IL-2R-bearing CD11+ suppressor monocytes.
Similar content being viewed by others
References
Smithers SR, Doenhoff MI: Schistosomiasis.In Immunology of parasitic infections, S Cohen, KS Warren (eds). Blackwell Scientific, Oxford, 1982, p 527
Bloom BR: Games parasites play. How parasites evade immune surveillance. Nature 279:21–26, 1979
Sebai ZA: Schistosomiasis in Saudi Arabia. Ann Saudi Med 3:269–174, 1988
Ottesen EA: Modulation of the host response in human schistosomiasis. I. Adherent suppressor cells that inhibit lymphocyte proliferative response to parasite antigen. J Immunol 123:1639–1646, 1979
Capron A, Dessain JP: Effector and regulatory mechanism in immunity to schistosomiasis. A heuristic review. Annu Rev Immunol 3:455–476, 1985
Warren KS: Hepatosplenic schistosomiasis mansoni. An immunologic disease. Bull NY Acad Med 51:545–554, 1975
Warren KS: The secret of immunopathogenesis of schistosomiasis: In vivo models. Immunol Rev 61:189–213, 1982
Mahmoud AAF: Current concepts: Schistosomiasis. Med Intell 297:1329–1332, 1977
Ellner JJ, Olds GR, Kamel R, Osman GS, El-Kholy A: Mahmoud AAF: Suppressor splenic T lymphocytes in human hepatosplenic schistosomiasis mansoni. J Immunol 125:308–311, 1980
Abdel-Tawab GA, Kelada FS, Kelada NI: Studies on the etiology of bilharzial cancer of the urinary bladder. Int J Cancer 1:377–382, 1966
Ezzat E, Osman RA, Ahmed KY, Soothil JF: The association between schistosoma haematobium infection and heavy proteinurea. Trans Roy Soc Trop Med Hyg 68:315–318, 1974
Raziuddin S, Shetty S, Ibrahim A, Patil K: Activated CD4+ T lymphocytes and impaired cell mediated immunity in patients of carcinoma of the urinary bladder with schistosomiasis. Cancer 65:931–939, 1990
Boros DL, Warren KS: Delayed hypersensitivity type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from schistosoma mansoni eggs. J Exp Med 132:488–507, 1970
Carter CE, Colley DG: An electrophoretic analysis of schistosoma mansoni egg antigen preparation. J Parasitol 64:385–390, 1978
Raziuddin S, Hussain NK, Latif ABA: A monoclonal antibody and functional study of malignant T cells of a patient with suppressor T cell lymphoma. Clin Immunol Immunopathol 45:230–234, 1987
Wysocki L, Sato VL: Panning of lymphocytes. A method for cell selection. Proc Natl Acad Sci USA 75:2844–2847, 1978
Raziuddin S, Kibler RF, Morrison DC: Immunosuppression of experimental allergic encephalomyelitis. III.In vitro evidence for induction of suppressor T lymphocytes in lymph nodes of animals immunized with myelin basic protein complexed to lipopolysaccharides. J Immunol 128:2073–2080, 1982
Mittler R, Rao P, Olini G, Westberg E, Newman W, Goldstein G: Activated human B cells display a functional IL-2 receptor. J Immunol 134:2393–2397, 1985
Herrmann F, Cannistra SA, Levine H, Griffin JD: Expression of interleukin-2 receptors and binding of interleukin-2 by gamma interferon-induced human leukemic and normal monocytic cells. J Exp Med 162:1111–1116, 1985
Holter W, Grunow R, Stockinger H, Knapp W: Recombinant interferon-y induces interleukin 2 receptors on human peripheral blood lymphocytes. J Immunol 136:2171–2175, 1986
Smith KA: Interleukin 2. Annu Rev Immunol 2:319–333, 1984
Stobo JD: Immunosuppression in man: Suppression by macrophages can be mediated by interactions with regulatory T cells. J Immunol 119:918–923, 1977
Gazzinelli G, Lambertucci JR, Katz N, Rocha RS, Lima MS, Colley DG: Immune responses during human schistosomiasis mansoni. XI. Immunologic status of patients with acute infections and after treatment. J Immunol 135:2121–2127, 1985
Luft BJ, Pedrott PW, Remington JS: In vitro generation of adherent mononuclear cells to toxoplasma antigen. Immunology 63:643–648, 1988
Piessens WS, Rattiwanto S, Tuti S, Palmieri JK, Piessens PW, Koiman I, Dennis DT: Antigen specific suppressor cells and suppressor factors in human filariasis with brugia malayi. N Engl J Med 302:333–338, 1980
Peterson EA, Neva FA, Barral A, Lorrea-Cornas R, Bogaeart-Diaz H, Martinoz D, Ward EE: Monocyte suppression of antigen-specific response in diffuse cutaneous leishmaniasis patients from the Dominican Republic. J Immunol 132:2603–2611, 1984
Toosi Z, Kleinhenz ME, Ellner JJ: Defective interleukin 2 production and responsiveness in human pulmonary tuberculosis. J Exp Med 163:1162–1172, 1986
Mohageghpour N, Gilber RH, Larrick JL, Sasaki DT, Bernnan PJ, Engleman EC: Defective cell mediated immunity in leprosy. Failure of T cells from leprosy patients to respond to mycobacterium leprae is associated with defective expression of IL-2 receptors and is not reconstituted by interleukin 2. J Immunol 135:1443–1449, 1985
Arno JN, Toosi Z, Edmonds K, Ellner JJ, Schmidt D: Decreased IL-2 production during reactivation of recurrent oral herpes simplex infection. Clin Res 32:363A, 1984 (abstr)
Harel-Bellan A, Joskowicz M, Eisen H: Modification of T cell proliferation and interleukin 2 production in mice infected with trypanosoma cruzi. Proc Natl Acad Sci USA 80:3466–3469, 1983
Reiner NE, Finke JH: Interleukin 2 deficiency in murine leishmaniasis donovani and its relationship to depressed spleen cell response to phytohemagglutinin. J Immunol 131:1487–1491, 1983
Kierszenbaum F, Bettz LA: Decreased human IL-2 receptor expression due to a protozoan pathogen. Immunol Today 10:129–131, 1989
Maizel AL, Ford RJ, Lachman LB: Effect of IL-1 on human thymocytes and human purified T cells. J Exp Med 155:943–952, 1981
Mizel SB: Interleukin 1 and T cell activation. Immunol Rev 63:51–69, 1982
Rappaport RS, Dodge G: Prostaglandin E inhibits the production of human interleukin 2. J Exp Med 155:943–952, 1982
Walker C, Kristensen F, Bettens F, DeWeck AL: Lymphokine regulation of activated lymphocytes. I. Prostaglandin E2 induced inhibition of interleukin 2 production. J Immunol 130:1770–1776, 1983
Chouaib S, Welte K, Mertelsmann R, Dupont B: Prostaglandin E2 acts at two distinct pathway of T lymphocyte activation. Inhibition of IL-2 and down regulation of transferrin receptor expression. J Immunol 135:1172–1179, 1985
Waldmann TA: The structure, function and expression of interleukin 2 receptors on normal and malignant lymphocytes. Science 232:727–732, 1986
Rosenberg SA: The adoptive therapy of cancer using the transfer of activated lymphoid cells and IL-2. Semin Oncol 13:200–216, 1986
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raziuddin, S., Shetty, S. & Ibrahim, A. T-cell abnormality and defective interleukin-2 production in patients with carcinoma of the urinary bladder with schistosomiasis. J Clin Immunol 11, 103–113 (1991). https://doi.org/10.1007/BF00917746
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00917746