Abstract
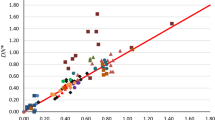
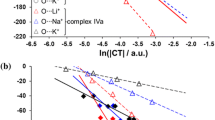
The electrophilic properties of 34 solvents have been characterized by the Acceptor Number (AN) which has been derived from31P-n.m.r. measurements of triethylphosphine oxide dissolved in the respective solvents. Relationships are found between the acceptor numbers and theZ-values,E T-values andY-values, as well as the free energies of solvation of anions and the redox potentials of the hexacyanoferrate(III)—hexacyanoferrrate(II) system in different solvents. The new parameter provides—together with the donor number—a useful guide in choosing the most appropriate solvent for a given reaction.
Similar content being viewed by others
References
V. Gutmann, Chemische Funktionslehre. Wien-New York: Springer. 1971.
V. Gutmann, Topics in Current Chem.27, 59 (1972).
V. Gutmann andR. Schmid, Coord. Chem. Rev.12, 263 (1974).
U. Mayer andV. Gutmann, Advan. Inorg. Chem. Radiochem.17, 189 (1975).
U. Mayer, Pure Appl. Chem.49, 291 (1975).
V. Gutmann andE. Wychera, Inorg. Nucl. Chem. Lett.2, 257 (1966).
V. Gutmann, Coordination Chemistry in Non-aqueous Solutions. Wien-New York: Springer. 1968.
R. Alexander, A. J. Parker, J. H. Sharp, andW. E. Waghorne, J. Amer. Chem. Soc.94, 1148 (1972).
B. G. Cox, G. R. Hedwig, A. J. Parker, andD. W. Watts, Austral. J. Chem.27, 477 (1974).
V. Gutmann, G. Gritzner, andK. Danksagmüller, Inorg. Chim. Acta (in the press).
R. H. Erlich andA. I. Popov, J. Amer. Chem. Soc.93, 5620 (1971).
P. Spaziante andV. Gutmann, Inorg. Chim. Acta5, 273 (1971).
E. A. Williams, Presented at the 9th Organosilicon Symposium in Cleveland, Ohio, April 5, 1975, private communication.
W. Gerger, U. Mayer, andV. Gutmann, in preparation.
F. Hein andH. Hecker, Chem. Ber.93, 1339 (1960).
A. Cahours andA. W. Hofmann, Ann. Chem.104, 23 (1857).
E. L. Gefter, Zhur. Obsh. Khim.28, 1338 (1958); Chem. Abstr.52, 19999d.
J. A. Riddick andW. B. Bunger, Techniques of Chemistry (A. Weissberger, ed.), Vol. II. New York-Toronto: Wiley-Interscience. 1970.
A. BAlasubramanian andC. N. R. Rao, Spectrochim. Acta18, 1337 (1962).
T. Olsen, Acta Chem. Scand.24, 3081 (1970).
U. Mayer andV. Gutmann, Structure and Bonding12, 113 (1972).
J. A. Creighton andK. M. Thomas, J. Chem. Soc., Dalton Trans.1972, 403.
H. Normant, Angew. Chem.79, 1029 (1967).
Ch. Reichardt, Lösungsmittel-Effekte in der organischen Chemie. Weinheim: Verlag Chemie. 1969.
E. M. Kosower, J. Amer. Chem. Soc.80, 3253 (1958).
K. Dimroth, Ch. Reichardt, T. Siepmann, andF. Bohlmann, Ann. Chem.661, 1 (1963).
E. Grunwald andS. Winstein, J. Amer. Chem. Soc.70, 846 (1948).
S. G. Smith, A. H. Fainberg, andS. Winstein, J. Amer. Chem. Soc.83, 618 (1961).
E. M. Kosower, J. Amer. Chem. Soc.80, 3261, 3267 (1958).
Ch. Reichardt andK. Dimroth, Topics in Current Chem.11, 1 (1968/69).
U. Mayer andV. Gutmann, Mh. Chem.101, 912 (1970).
V. Gutmann andU. Mayer, Mh. Chem.100, 2048 (1969).
V. Gutmann, Coord. Chem. Rev., submitted for publication.
V. Gutmann, Coord. Chem. Rev.15, 207 (1975).
V. Gutmann, Structure and Bonding15, 141 (1973).
S. D. Ross andM. M. Labes, J. Amer. Chem. Soc.79, 4155 (1957).
A. H. Fainberg andS. Winstein, J. Amer. Chem. Soc.78, 2770 (1956).
U. Mayer, Mh. Chem., to be published.
E. R. Swart andL. J. Le Roux, J. Chem. Soc.1957, 406.
J. J. Delpuech, Tetrahedron Letters25, 2111 (1965).
Ch. K. Jørgensen, J. Inorg. Nucl. Chem.24, 1587 (1962).
G. E. Maciel andJ. J. Natterstad, J. Chem. Phys.42, 2752 (1965).
D. J. Cram, B. Rickborn, C. A. Kingsbury, andP. Haberfield, J. Amer. Chem. Soc.83, 3678 (1961).
J. Miller andA. J. Parker, J. Amer. Chem. Soc.83, 117 (1961).
A. J. Parker, J. Chem. Soc.1961, 1328.
A. J. Parker, Advances in Physical Organic Chemistry (V. Gold, ed.), Vol. 5, p. 173. London-New York: Academic Press. 1967.
Author information
Authors and Affiliations
Additional information
With 8 Figures
Rights and permissions
About this article
Cite this article
Mayer, U., Gutmann, V. & Gerger, W. The acceptor number — A quantitative empirical parameter for the electrophilic properties of solvents. Monatshefte für Chemie 106, 1235–1257 (1975). https://doi.org/10.1007/BF00913599
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00913599