Abstract
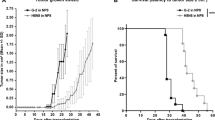
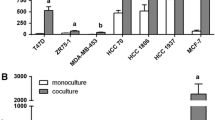
We have examined tumor progression and metastatic properties of three clonal murine mammary tumor cell lines of recent origin (D2A1, D2.OR and D2.1). These lines were derived from spontaneous mammary tumors which originated from a D2 hyperplastic alveolar nodule (HAN) line. D2A1 cells were more malignant than D2.OR or D2.1 cells, whether measured by experimental metastasis assays after intravenous injection in nude mice or chick embryos,in vivo growth rate of primary tumors following mammary fat pad injection in nude mice, or spontaneous metastasis assay from primary tumors growing in mammary fat pads. D2A1 cells also were more invasivein vitro in a Matrigel invasion assay than D2.1 cells, while the D2.OR cells were non-invasive in this assay. The increased invasiveness and malignancy of D2A1 cells were associated with increased levels of mRNA for the cysteine proteinase cathepsin L. Levels of osteopontin (OPN), nm23, int-1 and int-2 mRNAs were also examined. Nm23 levels were highest in the most malignant cell line. These cell lines provide a model for studying the tumorigenic and metastatic ability of mammary tumor cells and offer several advantages: they were cloned from mammary tumors that originate from a common source of preneoplastic cells (D2HAN); they are of relatively recent origin; and they have spontaneously arrived at different stages of tumor progression.
Similar content being viewed by others
References
Vaage J, 1988, Metastasizing potentials of mouse mammary tumors and their metastases.International Journal of Cancer,41, 855–858.
Frost P, Kerbel R, Hunt B, Man S and Pathak S, 1987, Selection of metastatic variants with identifiable karyotypic changes from a nonmetastatic murine tumor after treatment with 2-deoxy-5-azacytidine or hydroxyurea: implications for the mechanisms of tumor progression.Cancer Research,47, 2690–2695.
Waghorne C, Kerbel RS and Breitman ML, 1987, Metastatic potential of SPl mouse mammary adenocarcinoma cells is differentially induced by activated and normal forms of c-H-ras.Oncogene,1, 149–155.
Miller F, McEachern D and Miller B, 1989, Growth regulation of mouse mammary tumor cells in collagen gel cultures by diffusible factors produced by normal mammary gland epithelium and stromal fibroblasts.Cancer Research,49, 6091–6097.
Rak JW, McEachern D and Miller FR, 1992, Sequential alteration of peanut agglutinin binding-glycoprotein expression during progression of murine mammary neoplasia.British Journal of Cancer,65, 641–648.
Harkness MN, Bern HA, Alfert M and Goldstein NO, 1957, Cytochemical studies of hyperplastic alveolar nodules in the mammary gland of the C3H/He CRGL mouse.Journal of the National Cancer Institute,19, 1023–1029.
Pitelka DR, Bern HA, Deome KB, Schooley CN and Wellings SR, 1958, Virus-like particles in hyperplastic alveolar nodules of the mammary gland of the C3H/He CRGL mouse.Journal of the National Cancer Institute,20, 541–553.
Pitelka DR, Deome KB and Bern HA, 1960, Virus-like particles in precancerous hyperplastic mammary tissues of C3H and C3Hf mice.Journal of the National Cancer Institute,25, 753–777.
Wellings SR, Deome KB and Pitelka DR, 1960, Electron microscopy of milk secretion in the mammary gland of the C3H/Crg1 mouse, 1. Cytomorphology of the prelacting and the lactating gland.Journal of the National Cancer Institute,25, 393–421.
Medina D. Preneoplastic lesions in mouse mammary tumorigenesis. In: Busch H, ed.Methods in Cancer Research, pp. 3–53. New York: Academic Press, 1973.
McGrath CM and Jones RF, 1978, Hormonal induction of mammary tumor viruses and its implications for carcinogenesis.Cancer Research,38, 4112–4125.
Cardiff RD, 1984, Protoneoplasia: the molecular biology of murine mammary hyperplasia.Advances in Cancer Research,42, 167–190.
Nusse R, Van Ooyen A, Cox D, Fung YKT and Varmus H, 1984, Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15.Nature,307, 131–136.
Peters G, Lee AE and Dickson C, 1984, Activation of cellular gene by mouse mammary tumor virus may occur early in mammary tumor development.Nature 309, 273–275.
Jackson DP, Percy DH and Morris VL, 1984, Characterization of murine hepatitis virus (JHM) RNA from rats with experimental encephalomyelitis.Virology,137, 297–304.
Tuck AB, Wilson SM, Khokha R and Chambers AF, 1991, Different patterns of gene expression in ras-resistant andras-sensitive cells.Journal of the National Cancer Institute,83, 485–491.
Hill RP, Chambers AF, Ling V and Harris JF, 1984, Dynamic heterogeneity: rapid generation of metastatic variants in mouse B16 melanoma cells.Science,224, 998–1001.
Chambers AF, Shafir R and Ling V, 1982, A model system for studying metastasis using the embryonic chick.Cancer Research,42, 4018–4025.
Chambers AF, Wilson SM, Tuck AB, Denhardt GH and Cairncross JG, 1990, Comparison of metastatic properties of a variety of mouse, rat, and human cells in assays in nude mice and chick embryos.In Vivo,4, 215–220.
Auffray C and Rougeon F, 1980, Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA.European Journal of Biochemistry,107, 303–314.
Craig AM, Bowden GT, Chambers AF, Spearman MA, Greenberg AH, Wright JA, McLeod M and Denhardt DT, 1990, Secreted phosphoprotein mRNA is induced during multi-stage carcinogenesis in mouse skin and correlates with the metastatic potential of murine fibroblasts.International Journal of Cancer,46, 133–137.
Denhardt DT, Hamilton RT, Parfett CLJ, Edwards DR, St Pierre R, Waterhouse P and Nilsen-Hamilton M, 1986, Close relationship of the major excreted protein of transformed murine fibroblasts to thiol-dependent cathepsins.Cancer Research,46, 4590–4593.
Steeg PS, Bevilacqua G, Kopper L, Thorgeirsson UP, Talmadge JE, Liotta LA and Sobel ME, 1988, Evidence for a novel gene associated with low tumor metastatic potential.Journal of the National Cancer Institute,80, 200–204.
Cohen JC, Shank PR, Morris VL, Cardiff R and Varmus HE, 1979, Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissues of the mouse.Cell,16, 333–345.
Minty AJ, Alonso S, Guenet J-L and Buckingham ME, 1983, Number and organization of actin-related sequences in the mouse genome.Journal of Molecular Biology,167, 77–101.
Miller FR, Medina D and Heppner GH, 1981, Preferential growth of mammary tumors in intact mammary fat pads.Cancer Research,41, 3863–3867.
Miller FR, 1981, Comparison of metastasis of mammary tumors growing in the mammary fat pad versus subcutis.Invasion and Metastasis,1, 220–226.
Chambers AF, Colella R, Denhardt DT and Wilson SM, 1992, Increased expression of cathepsins L and B and decreased expression of their inhibitor in metastatic,ras-transformed NIH 3T3 cells.Molecular Carcinogenesis,5, 238–245.
Denhardt DT, Greenberg AH, Egan SE, Hamilton RT and Wright JA, 1987, Cysteine proteinase cathepsin L expression correlates closely with the metastatic potential of H-ras-transformed murine fibroblasts.Oncogene,2, 55–59.
Bevilacqua G, Sobel ME, Liotta LA and Steeg PS, 1989, Association of low nm23 RNA levels in human primary infiltrating ductal breast carcinomas wih lymph node involvement and other histopathological indicators of high metastatic potential.Cancer Research,49, 5185–5190.
Haut M, Steeg P, Willson J and Markowitz S, 1991, Induction of nm23 gene expression in human colonic neoplasms and equal expression in colon tumors of high and low metastatic potential.Journal of the National Cancer Institute,83, 712–716.
Lacombe ML, Sastre-Garau X, Lascu I, Vonica A, Wallet V, Thiery JP and Veron M, 1991, Overexpression of nucleoside diphosphate kinase (Nm23) in solid tumors.European Journal of Cancer,27, 1302–1307.
Dearolf C, Hersperger E and Shearn A, 1988, Developmental consequences ofawdb3, a cell-autonomous lethal mutation ofDrosophila induced by hybrid dysgenesis.Developmental Biology,129, 159–168.
Rosengard A, Krutzsch H, Shearn A, Biggs J, Barker E, Margulies I, King C, Liotta L and Steeg P, 1989, ReducedNm23/Awd protein in tumor metastasis and aberrantDrosophila development.Nature,342, 177–180.
Steeg PS, Cohn KH and Leone A, 1991, Tumor metastasis and nm23: current concepts.Cancer Cells,3, 257–262.
Craig A and Denhardt D, 1991, The murine gene encoding secreted phosphoprotein 1 (osteopontin): promoter structure, activity, and inductionin vivo by estrogen and progesterone.Gene,100, 163–171.
Craig AM, Smith JH and Denhardt DT, 1989, Osteopontin, a transformation-associated cell adhesion phosphoprotein, is induced by 12-O-tetrade canoylphorbol-13-acetate in mouse epidermis.Journal of Biological Chemistry,264, 9682–9689.
Oldberg A, Franzen A and Heinegard D, 1986, Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence.Proceedings of the National Academy of Sciences,83, 8819–8823.
Prince CW, Oosawa T, Butler WT, Tomana M, Bhown AS and Schrohenloher RE, 1987, Isolation, characterization, and biosynthesis of a phosphorylated glycoprotein from rat bone.Journal of Biological Chemistry,262, 2900–2907.
Mark MP, Prince CW, Gay S, Austin RL and Butler WR, 1987, Immunohistochemical demonstration of a 44-kd phosphoprotein in developing rat bones.Journal of Histochemistry and Cytochemistry,35, 707–715.
Nomura S, Wills AJ, Edwards DR, Heath JK and Hogan BL, 1988, Developmental expression of tar (osteopontin) and SPARC (osteopontin) RNA as revealed byin situ hybridization.Journal of Cell Biology,106, 441–450.
Waterhouse P, Parhar RS, Guo X, Lala PK and Denhardt DT, 1992, Regulated temporal and spatial expression of the calcium-binding proteins calcyclin and OPN (osteopontin) in mouse tissues during pregnancy.Molecular Reproduction and Development,32, 315–323.
Chambers AF, Behrend EI, Wilson SM and Denhardt DT, 1992, Induction of expression of osteopontin (OPN; secreted phosphoprotein) in metastatic,ras-transformed NIH 3T3 cells.Anticancer Research,12, 43–48.
Senger DR, Asch BB, Smith BD, Perruzzi CA and Dvorak HF, 1983, A secreted phosphoprotein marker for neoplastic transformation of both epithelial and fibroblastic cells.Nature,302, 714–715.
Senger D, Perruzzi CA and Papadopoulos A, 1989, Elevated expression of secreted phosphoprotein I (osteopontin, 2ar) as a consequence of neoplastic transformation.Anticancer Research,9, 1291–1300.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Morris, V.L., Tuck, A.B., Wilson, S.M. et al. Tumor progression and metastasis in murine D2 hyperplastic alveolar nodule mammary tumor cell lines. Clin Exp Metast 11, 103–112 (1993). https://doi.org/10.1007/BF00880071
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00880071