Abstract
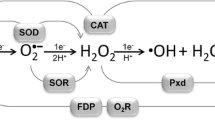
Oxygen is favoured as terminal electron acceptor in aerobic and facultative microorganisms because of its appropriate physical state, satisfactory solubility and its desirable combinations of kinetic and thermodynamic properties. Oxygen is generally reduced by four electrons to yield oxygen, but there are important biological consequences of, and roles for, the partial reduction to superoxide and peroxide. Complex and multiple regulatory networks ensure (i) the utilization of oxygen in preference to other oxidants, (ii) the synthesis of oxygen-consuming enzymes with appropriate properties (particularly affinity for the ligand), and (iii) appropriate cellular protection in the event of oxidative stress. This contribution reviews the terminal respiratory oxidases of selected Gram-negative bacteria and microbial haemoglobin-like proteins.
Recent studies of the cytochromebd-type oxidases ofEscherichia coli andAzotobacter vinelandii suggest that, despite probable similarity at the amino acid level, the reactivities of these oxidases with oxygen are strikingly different. The respiratory protection afforded to nitrogenase in the obligately aerobic diazotrophA. vinelandii by the cytochromebd complex appears to be accompanied by, and may be the result of, a low affinity for oxygen and a high Vmax. The poorly characterized cytochromeo-containing oxidase in this bacterium is not required for respiratory protection. InE. coli, the cytochromebd-type oxidase has a remarkably high affinity for oxygen, consistent with the view that this is an oxygen-scavenging oxidase utilized under microaerobic conditions. The demonstration of substrate (i.e. oxygen) inhibition in this complex suggests a mechanism whereby wasteful electron flux through a non-proton-pumping oxidase is avoided at higher dissolved oxygen tensions. The demonstration of two ligandbinding sites (haemsd andb 595) in oxidases of this type suggests plausible mechanisms for this phenomenon. InE. coli, assembly of the cytochromebd-type oxidase (and of periplasmic cytochromesb andc) requires the presence of an ABC transporter, which may serve to export haem or some “assembly factor' to the periplasm.
There is at least one additional oxygen-consuming protein inE. coli — the flavohaemoglobin encoded by thehmp gene. Globin-like proteins are also widely distributed in other bacteria, fungi and protozoa, but most have unknown functions. The function of HMP and the related chimaeric flavohaemoglobins in other bacteria and yeast is unknown; one of several possibilities for HMP is that its relatively low affinity for oxygen during turnover with NADH as substrate could enable it to function as a sensor of falling (or rising) cytoplasmic oxygen concentrations.
Similar content being viewed by others
References
Andrews SC, Shipley D, Keen JN, Findlay JBC, Harrison PM & Guest JR (1992) The haemoglobin-like protein (HMP) ofEscherichia coli has ferrisiderophore reductase activity and its C-terminal domain shares homology with ferredoxin NADP+ reductases. FEBS Lett. 302: 247–252
Anraku Y (1988) Bacterial electron transport chains. Ann. Rev. Biochem. 57: 101–132
Appleby CA (1969) Electron transport systems ofRhizobium japonicum II.Rhizobium haemoglobin, cytochromes and oxidases in free-living (cultured) cells. Biochim. Biophys. Acta 172: 88–105
Appleby CA (1984) Leghaemoglobin andRhizobium respiration. Ann. Rev. Plant Physiol. 35: 443–478
Appleby CA, Bogusz D, Dennis ES & Peacock WJ (1988) A role for haemoglobin in all plant roots? Plant Cell Environ. 11: 359–367
Appleby CA, Dennis ES & Peacock WJ (1990) A primaeval orgin for plant and animal haemoglobins? Aust. Syst. Bot. 3: 81–89
Bebbington KJ & Williams HD (1993) Investigation of the role of thecydD gene product in production of a functional cytochromed oxidase inEscherichia coli. FEMS Microbiol. Lett. 112: 19–24
Becana M, Salin ML, Ji L & Klucas RV (1991) Flavin-mediated reduction of ferric leghemoglobin from soybean nodules. Planta 183: 575–583
Bergerson FJ & Turner GL (1979) Systems utilizing oxygenated leghaemoglobin and myoglobin as sources of free dissolved oxygen at low concentrations for experiments with bacteria. Anal. Biochem. 96: 165–174
Bolgiano B, Salmon I, Ingledew WJ & Poole RK (1991) Redox analysis of the cytochromeo-type quinol oxidase complex ofEscherichia coli reveals three redox components. Biochem. J. 274: 723–730
Calhoun MW, Thomas JW, Hill JJ, Hosler JP, Shapleigh JP, Tecklenburg MMJ, Ferguson-Miller S, Babcock GT, Alben JO & Gennis RB (1993) Identity of the axial ligand of the high-spin heme in cytochrome oxidase. Spectroscopic characterization of mutants in thebo-type oxidase ofEscherichia coli and theaa 3-type oxidase ofRhodobacter sphaeroides. Biochemistry 32: 10905–10911
Castor LN & Chance B (1959) Photochemical determinations of the oxidases of bacteria. J. Biol. Chem. 234: 1587–1592
Chance B (1989) Commentary on ‘New methods for the study of the carbon monoxide compounds of respiratory enzymes’. Biochim. Biophys. Acta 1000: 345–347
Chen L, Liu M-Y, Legall J, Faraleira P, Santos H & Xavier AV (1993) Rubredoxin oxidase, a new flavo-hemo-protein, is the site of oxygen reduction to water by the “strict anaerobe”Desulfovibrio gigas. Biochem. Biophys. Res. Comm. 193: 100–105
Chepuri V, Lemieux L, Au DCT and Gennis RB (1990) The sequence of thecyo operon indicates substantial structural similarities between the cytochrome-o ubiquinol oxidase ofEscherichia coli and theaa 3-type family of cytochrome-c oxidases. J. Biol. Chem. 265: 11185–11192
Ciccognani DT, Hughes MN & Poole RK (1992) Carbon monoxide-binding properties of the cytochromebo quinol oxidase complex inEscherichia coli are changed by copper deficiency in continuous culture. FEMS Microbiol. Lett. 94: 1–6
Cooper CE, Ioannidis N, D'mello R & Poole RK (1994) Haem, flavin and oxygen interactions in Hmp, a flavohaemoglobin fromEscherichia coli. Biochem. Soc. Trans. (in press)
Cotter PA, Chepuri V, Gennis RB & Gunsalus RP (1990) Cytochromeo (cyoABCDE) andd (cydAB) oxidase gene expression is regulated by oxygen, pH, and thefnr gene product. J. Bacteriol. 172: 6333–6338
Cramm R, Siddiqui RA & Friedrich B (1994) Primary sequence and evidence for a physiological function of the flavohemoprotein ofAlcaligenes eutrophus. J. Biol. Chem. 269: 7349–7354
D'mello R, Palmer S, Hill S & Poole RK (1994a) The cytochromebd terminal oxidase ofAzotobacter vinelandii: low temperature photodissociation spectrophotometry reveals reactivity of cytochromesb 595 andd with both carbon monoxide and oxygen. FEMS Microbiol. Lett. (submitted)
D'mello R, Hill S & Poole RK (1994b) Determination of the oxygen affinities of terminal oxidases inAzotobacter vinelandii using the deoxygenation of oxyleghaemoglobin and oxymyoglobin: cytochromebd is a low-affinity oxidase. Microbiology (in press)
Delaney JM & Georgopoulos C (1992) Physical map locations of thetrxB, htrD, cydC, andcydD genes ofEscherichia coli. J. Bacteriol. 174: 3824–3825
Delaney JM, Wall D & Georgopoulos C (1993) Molecular characterization of theEscherichia coli htrD gene: cloning, sequence, regulation, and involvement with cytochromed oxidase. J. Bacteriol. 175: 166–175
Dikshit KL & Webster DA (1988) Cloning, characterization and expression of the bacterial globin gene fromVitreoscilla inEscherichia coli. Gene 70: 377–386.
Dikshit KL, Spaulding D, Braun A & Webster DA (1989) Oxygen inhibition of globin gene transcription and bacterial haemoglobin synthesis inVitreoscilla. J. Gen. Microbiol. 135: 2601–2609
Dikshit RP, Dikshit KL, Liu Y & Webster DA (1992) The bacterial haemoglobin fromVitreoscilla can support the aerobic growth ofEscherichia coli lacking terminal oxidases. Archiv. Biochem. Biophys. 293: 241–245
Eschenbrenner M, Coves J & Fontecave M (1994) Ferric reductases inEscherichia coli: the contribution of the haemoglobin-like protein. Biochem. Biophys. Res. Comm. 198: 127–131
Fang H, Lin R-J & Gennis RB (1989) Location of heme axial ligands in the cytochromed terminal oxidase complex ofEscherichia coli determined by site-directed mutagenesis. J. Biol. Chem. 264: 8026–8032
Fath MJ & Kolter R (1993) ABC transporters: bacterial exporters. Microbiol. Rev. 57: 995–1017
Fu R, Wall JD & Voordouw G (1994) DcrA, ac-type hemecontaining methyl-accepting protein fromDesulfovibrio vulgaris Hildenborough, senses the oxygen concentration or redox potential of the environment. J. Bacteriol. 176: 344–350
Georgiou CD, Fang H & Gennis RB (1987) Identification of thecydC locus required for expression of the functional form of the cytochromed terminal oxidase complex inEscherichia coli. J. Bacteriol. 169: 2107–2112
Gibson QH (1989) Hemoproteins, ligands, and quanta. J. Biol. Chem. 264: 20155–20158
Gilles-Gonzalez MA, Ditta GS & Helinski DR (1991) A haemoprotein with kinase activity encoded by the oxgen sensor ofRhizobium meliloti. Nature 350: 170–172
Goldberg MA, Dunning SP & Bunn HF (1988) Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science 242: 1412–1415
Green GN, Fang H, Lin R-J, Newton G, Mather M, Georgiou CD & Gennis RB (1988) The nucleotide sequence of thecyd locus encoding the two subunits of the cytochromed terminal oxidase complex ofEscherichia coli. J. Biol. Chem. 263: 13138–13143
Green J & Guest JR (1993) Activation of FNR-dependent transcription by iron: an in vitro switch for FNR. FEMS Microbiol. Lett. 113: 219–222
Haddock BA & Jones CW (1977) Bacterial respiration. Bacteriol. Rev. 41: 47–99
Higgins CF (1992) ABC transporters: from microorganisms to man. Ann. Rev. Cell Biol. 8: 67–113
Hill J, Goswitz VC, Calhoun M, Garcia-Horsman JA, Lemieux L, Alben JO & Gennis RB (1992) Demonstration that thebo-type ubiquinol oxidase ofEscherichia coli contains a heme-copper binuclear center similar to that in cytochromec oxidase and that proper assembly of the binuclear centre requires thecyoE gene product. Biochemistry 31: 11435–11440
Hill JJ, Alben JO & Gennis RB (1993) Spectroscopic evidence for a heme-heme binuclear center in the cytochromebd ubiquinol oxidase fromEscherichia coli. Proc. Natl. Acad. Sci. USA 90: 5863–5867
Hill S, Viollet S, Smith AT & Anthony C (1990) Roles for entericd-type cytochrome oxidase in N2 fixation and microaerobiosis. J. Bacteriol. 172: 2071–2078.
Hussain H, Grove J, Griffiths L, Busby S & Cole JA (1994) A sevengene operon essential for formate-dependent nitrite reduction to ammonia by enteric bacteria. Mol. Microbiol. 12: 153–163
Ioannidis I, Cooper C & Poole RK (1992) Spectroscopic studies on an oxygen-binding haemoglobin-like flavohaemoprotein fromEscherichia coli. Biochem. J. 288: 649–655
Iuchi S (1993) Phosphorylation/dephosphorylation of the receiver module at the conserved aspartate residue controls transphosphorylation activity of histidine kinase in sensor protein ArcB ofEscherichia coli. J. Biol. Chem. 268: 23972–23980
Iuchi S & Lin ECC (1993) Adaptation ofEscherichia coli to redox environments by gene expression. Mol. Microbiol. 9: 9–15
Iwaasa H, Takagi T & Shiama K (1990) Protozoan hemoglobin fromTetrahymena pyriformis. Isolation, characterization, and amino acid sequence. J. Biol. Chem. 265: 8603–8609
Iwaasa H, Takagi T & Shikama K (1992) Amino acid sequence of yeast hemoglobin. A two-domain structure. J. Mol. Biol. 227: 948–954
Iwasaki H (1966) Lactate oxidation system inAcetobacter suboxydans, with special reference to carbon monoxide-binding pigment. Plant Cell Physiol. 7: 199–216
Jakob W, Webster DA & Kroneck PMH (1992) NADH-dependent methemoglobin reductase from the obligate aerobeVitreoscilla: improved method of purification and reexamination of prosthetic groups. Arch. Biochem. Biophys. 292: 29–33
Kahlow MA, Zuberi TM, Gennis RB & Loehr TM (1991) Identification of a ferryl intermediate ofEscherichia coli cytochromed terminal oxidase by resonance Raman spectroscopy. Biochemistry 30: 11485–11489
Kahn D (1993) Shuffling of an oxygen sensor haem domain. Mol. Microbiol. 8: 786–787
Karplus PA, Daniels MJ & Herriott JR (1991) Atomic structure of ferredoxin-NADP+ reductase: prototype for a structurally novel flavoenzyme family. Science 251: 60–66
Keilin D (1953) Occurrence of haemoglobin in yeast and the supposed stabilization of the oxygenated cytochrome oxidase. Nature 172: 390–393
Keilin D & Tissières A (1953) Haemoglobin in moulds:Neurospora crassa andPenicillium notatum. Nature 172: 393–394
Keilin D (1966) The History of Cell Respiration and Cytochrome. Cambridge University Press, Cambridge
Kelly MJS, Poole RK, Yates MG & Kennedy C (1990) Cloning and mutagenesis of genes encoding the cytochromebd complex inAzotobacter vinelandii. Mutants deficient in cytochromed are unable to fix nitrogen in air. J. Bacteriol. 172: 6010–6019
Khosla C & Bailey JE (1988) Heterologous expression of a bacterial haemoglobin improves the growth properties of recombinantEscherichia coli. Nature 331: 633–635
Khosla C & Bailey JE (1989a) Evidence for partial export ofVitreoscilla hemoglobin into the periplasmic space inEscherichia coli. Implications for protein function. J. Mol. Biol. 210: 79–89
Khosla C & Bailey JE (1989b) Characterization of the oxygen-dependent promoter of theVitreoscilla hemoglobin gene inEschericia coli. J. Bacteriol. 171: 5995–6004
Krasnoselskaya I, Arutjunjan AM, Smirnova I, Gennis R & Konstantinov AA (1993) Cyanide-reactive sites in cytochromebd complex fromE. coli. FEBS Lett. 327: 279–283
Leung D, van der Oost J, Kelly M, Saraste M, Hill S & Poole RK (1994) Mutagenesis of a gene encoding a cytochromeo-like terminal oxidase ofAzotobacter vinelandii: a cytochromeo mutant is aero-tolerant during nitrogen fixation. FEMS Microbiol. Lett. (in press)
Light WR & Olson JS (1990) Transmembrane movement of haem. J. Biol. Chem. 265: 15623–15631
Lorence RM & Gennis RB (1989) Spectroscopic and quantitative analysis of the oxygenated and peroxy states of the purified cytochromed complex ofEscherichia coli. J. Biol. Chem. 264: 7135–7140
Lorence RM, Koland JG & Gennis RB (1986) Coulometric and spectroscopic analysis of the purified cytochromed complex ofEscherichia coli: evidence for the identification of “cytochromea 1” as cytochromeb 595. Biochemistry 25: 2314–2321
Minghetti KC, Goswitz VC, Gabriel NE, Hill JJ, Barassi CA, Georgiou CD, Chan SI & Gennis RB (1992) Modified, large-scale purification of the cytochromeo complex (bo-type oxidase) ofEscherichia coli yields a two heme/one copper terminal oxidase with high specific activity. Biochemistry 31: 6917–6924
Monson EK, Weinstein M, Ditta GS & Helinski DR (1992) The FixL protein ofRhizobium meliloti can be separated into a hemebinding oxygen-sensing domain and a functional C-terminal kinase domain. Proc. Natl. Acad. Sci. USA 89: 4280–4284
Morgan JE, Verkhovsky MI, Puustinen A & Wikström M (1993) Intramolecular electron transfer in cytochromeo ofEscherichia coli: events following the photolysis of fully and partially reduced CO-bound forms of thebo 3 andoo 3 enzymes. Biochemistry 32: 11413–11418
Moshiri F, Chawla A & Maier RJ (1991) Cloning, characterization, and expression inEscherichia coli of the genes encoding the cytochromed oxidase complex fromAzotobacter vinelandii. J. Bacteriol. 173: 6230–6241
Orii Y, Ioannidis N & Poole RK (1992) The oxygenated flavohaemoglobin fromEscherichia coli: evidence from photodissociation and rapid-scan studies for two oxygenated forms. Biochem. Biophys. Res. Commun. 187: 94–100
Oshino R, Oshino N, Chance B & Hagihara B (1973a) Studies on yeast hemoglobin. The properties of yeast hemoglobin and its physiological function in the cell. Eur. J. Biochem. 35: 23–33
Oshino R, Asakura T, Takio K, Oshino N, Chance B & Hagihara B (1973b) Purification and molecular properties of yeast hemoglobin. Eur. J. Biochem. 39: 581–590
Perutz MF (1986) A bacterial haemoglobin. Nature 322: 405
Poole RK (1983) Bacterial cytochrome oxidases: a structurally and functionally diverse group of electron transfer proteins. Biochim. Biophys. Acta 726: 205–283
Poole RK (1988) Bacterial cytochrome oxidases. In: Anthony C (ed) Bacterial Energy Transduction (pp 231–291) Academic Press, London
Poole RK & Ingledew WJ (1987)Escherichia coli andSalmonella typhimurium. In: Ingraham JL, Low KB, Magasanick B, Schaechter M and Umbarger H (eds) Cellular and Molecular Biology, Vol. 1 (pp 170–200) American Society for Microbiology, Washington, DC
Poole RK & Williams HD (1988). Formation of the 680 nmabsorbing form of the cytochromebd oxidase complex ofEscherichia coli by reaction of hydrogen peroxide with the oxidized form. FEBS Lett. 231: 243–246
Poole RK, Waring AJ & Chance B (1979) The reaction of cytochromeo inEscherichia coli with oxygen. Low-temperature kinetic and spectral studies. Biochem. J. 184: 379–389
Poole RK, Scott RI & Chance B (1981) The light-reversible binding of carbon monoxide to cytochromea 1 inEscherichia coli K12. J. Gen. Microbiol. 125: 431–438
Poole RK, Sivaram A, Salmon I & Chance B (1982) Photolysis at very low temperatures of CO-liganded cytochrome oxidase (cytochromed) in oxygen-limitedEscherichia coli. FEBS Lett. 141: 237–241
Poole RK, Kumar C, Salmon I & Chance B (1983) The 650 nm chromophore inEscherichia coli is an “oxy” — or oxygenated compound, not the oxidized form of cytochrome oxidased: a hypothesis. J. Gen. Microbiol. 129: 1335–1344
Poole RK, Williams HD, Downie JA & Gibson F (1989) Mutations affecting the cytochromed-containing oxidase complex ofEscherichia coli K12: Identification and mapping of a fourth locus,cydD. J. Gen. Microbiol. 135: 1865–1874
Poole RK, Hatch L, Cleeter M, Gibson F, Cox GB & Wu G (1993) Cytochromebd biosynthesis inEscherichia coli: the sequences of thecydC andcydD genes suggest that they encode the components of an ABC membrane transporter. Mol. Microbiol. 10: 421–430
Poole RK, Salmon I & Chance B (1994a) The high-spin cytochromeo′ component of the cytochromebo-type quinol oxidase in membranes fromEscherichia coli: formation of the primary oxygenated species at low temperature is characterized by a slow ‘on’ rate and low dissociation constant. Microbiology (in press)
Poole RK, Gibson F & Wu G (1994b) ThecydD gene product, component of a heterodimeric ABC transporter, is required for assembly of periplasmic cytochromec and of cytochromebd inEscherichia coli. FEMS Microbiol. Lett. 117: 217–224
Poole RK, D'mello R, Hill, S. Ioannidis I, Leung D & Wu G (1994c) The oxygen reactivity of bacterial respiratory haemoproteins: oxidases and globins. Biochim. Biophys. Acta (in press)
Poole RK, Ioannidis N & Orii Y (1994d) Reactions of theEscherichia coli flavohaemoglobin (Hmp) with oxygen and reduced nicotinamide adenine dinucleotide. Evidence for oxygen switching of flavin oxidoreduction and a mechanism for oxygen sensing. Proc. Roy. Soc. Ser. B 255: 251–258
Potts M, Angeloni SV, Ebel RE & Bassam D (1992) Myoglobin in a cyanobacterium. Science 256: 1690–1692
Preisig O, Anthamatten D & Hennecke H (1993) Genes for a microaerobically induced oxidase complex inBradyrhizobium japonicum are essential for a nitrogen-fixing endosymbiosis. Proc. Natl. Acad. Sci. 90: 3309–3313
Probst I, Wolf G, Schlegel HG & Fischer G (1979) An oxygen-binding flavohaemoprotein fromAlcaligenes eutrophus. Biochim. Biophys. Acta 576: 471–478
Puustinen A & Wikström M (1991) The haem groups of cytochromeo fromEscherichia coli. Proc. Natl. Acad. Sci. USA 88: 6122–6126
Puustinen A, Finel M, Haltia T, Gennis RB & Wikström M (1991) Properties of the two terminal oxidases fromEscherichia coli. Biochemistry 30: 3936–3942
Puustinen A, Morgan JE, Verkhovsky M, Thomas J W, Gennis RB & Wikström M (1992) The low-spin heme site of cytochromeo fromEscherichia coli is promiscuous with respect to heme type. Biochemistry 31: 10363–10369
Saiki K, Mogi T & Anraku Y (1992) Heme O biosynthesis inEscherichia coli: thecyoE gene in the cytochromebo operon encodes a protoheme IX farnesyltransferase. Biochem. Biophys. Res. Comm. 189: 1491–1497
Salerno JC, Bolgiano B, Poole RK, Gennis RB & Ingledew WJ (1990). Heme-copper and heme-heme interactions in the cytochromebo-containing quinol oxidase ofEscherichia coli. J. Biol. Chem. 265: 4364–4368
Saraste M (1994) Structure and evolution of cytochrome oxidase. Antonie van Leeuwenhoek (this issue) 65: 285–287
Siegele DA & Kolter R (1993) Isolation and characterization of anEscherichia coli mutant defective in resuming growth after starvation. Genes Dev. 7: 2629–2640
Stewart V (1993) Nitrate regulation of anaerobic respiratory gene expression inEscherichia coli. Mol. Microbiol. 9: 425–434
Svensson M & Nilsson T (1993) Flow-flash study of the reaction between cytochromebo and oxygen. Biochemistry 32: 5442–5447
Takagi T (1993) Hemoglobins from single-celled organisms. Curr. Opin. Struct. Biol. 3: 413–418
Thöny-Meyer L, Ritz & Hennecke H (1994) Cytochromec biogenesis in bacteria: a possible pathway begins to emerge. Mol. Microbiol. 12: 1–9
Timkovich R, Cork MS, Gennis RB & Johnson PY (1985) Proposed structure of hemed, a prosthetic group of bacterial terminal oxidases. J. Am. Chem. Soc. 107: 6069–6075
Unden G, Trageser M & Duchene A (1990) Effect of positive redox potentials (> + 400 mV) on the expression of anaerobic respiratory enzymes inEscherichia coli. Mol. Microbiol. 4: 315–319
van der Oost J, de Boer APN, de Gier J-WL, Zumft WG, Stouthamer AH & van Spanning R (1994) The heme-copper oxidase family consists of three distinct types of terminal oxidases and is related to nitric oxide reductase. FEMS Microbiol. Lett. (in press)
Vasudevan SG, Armarego WLF, Shaw DC, Lilley PE, Dixon NE & Poole RK (1991) Isolation and nucleotide sequence of thehmp gene that encodes a haemoglobin-like protein inEscherichia coli K-12. Mol. Gen. Genet. 226: 49–58
Verhoeven W (1956) Some remarks on nitrate and nitrite metabolism in microorganisms. In: McElroy WD and Glass B (eds) Inorganic Nitrogen Metabolism: Function of Metallo-Flavoproteins (pp61–86) Johns Hopkins Press, Baltimore
Wakabayashi S, Matsubara H & Webster DA (1986) Primary sequence of a dimeric bacterial haemoglobin fromVitreoscilla. Nature 322: 481–483
Webster DA (1987) Structure and function of bacterial hemoglobin and related proteins. In: Eichhorn GL and Marzilli LG (eds) Advances in Inorganic Chemistry, Vol 7, Haem Proteins (pp 245–265) Elsevier, New York
Wittenberg JB & Wittenberg BA (1990) Mechanisms of cytoplasmic hemoglobin and myoglobin function. Annu. Rev. Biophys. Biophys. Chem. 19: 217–241
Wood PM (1984) Bacterial proteins with CO-bindingb- orc-type haem. Functions and absorption spectroscopy. Biochim. Biophys. Acta 768: 293–317
Wu G, Williams HD, Gibson F & Poole RK (1993) Mutants ofEscherichia coli affected in respiration: the cloning and nucleotide sequence ofubiA, encoding the membrane-boundp-hydroxybenzoate:octaprenyl transferase. J. Gen. Microbiol. 139: 1795–1805
Xu F, Quandt KS & Hultquist DE (1992) Characterization of NADPH-dependent methemoglobin reductase as a heme-binding protein present in erythrocytes and liver. Proc. Natl. Acad. Sci. USA 89: 2130–2134
Yamauchi K, Ochiai T & Usuki I (1992) The unique structure of theParamecium caudatum hemoglobin gene: the presence of one intron in the middle of the coding region. Biochim. Biophys. Acta 1171: 81–87
Youngson C, Nurse C, Yeger H & Cutz E (1993) Oxygen sensing in airway chemoreceptors. Nature 365: 153–155
Zhu H & Riggs AF (1992) Yeast flavohemoglobin is an ancient protein related to globins and a reductase family. Proc. Natl. Acad. Sci. USA 89: 5015–5019
Author information
Authors and Affiliations
Additional information
(until October 1994: Section of Microbiology, Wing Hall, Cornell University, Ithaca, NY 14853-8101, USA)
Rights and permissions
About this article
Cite this article
Poole, R.K. Oxygen reactions with bacterial oxidases and globins: binding, reduction and regulation. Antonie van Leeuwenhoek 65, 289–310 (1994). https://doi.org/10.1007/BF00872215
Issue Date:
DOI: https://doi.org/10.1007/BF00872215