Abstract
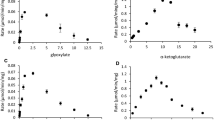
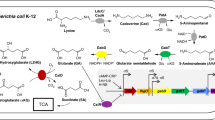
Some strict anaerobic bacteria catalyze with glycine as substrate an internal Stickland reaction by which glycine serves as electron donor being oxidized by glycine-cleavage system or as electron acceptor being reduced by glycine reductase. In both cases, energy is conserved by substrate level phosphorylation. Except for the different substrate-activating proteins P B , reduction of sarcosine or betaine to acetyl phosphate involves inEubacterium acidaminophilum the same set of proteins as observed for glycine, e.g. a unique thioredoxin system as electron donor and an acetyl phosphate-forming protein P c interacting with the intermediarily formed Secarboxymethylselenoether bound to protein P A .
Similar content being viewed by others
References
Andreesen JR (1994) Acetate via glycine: a different form of acetogenesis. In: Drake HL (Ed) Acetogenesis (pp 568–626). Chapman & Hall Inc., New York
Andreesen JR, Schaupp A, Neurauter C, Brown A & Ljungdahl LG (1973) Fermentation of glucose, fructose, and xylose byClostridium thermoaceticum: effect of metals on growth, yield, enzymes, and the synthesis of acetate from CO2. J. Bacteriol. 114: 743–751
Arkowitz RA & Abeles RH (1989) Identification of acetyl phosphate as the product of clostridial glycine reductase: evidence for an acyl enzyme intermediate. Biochemistry 28: 4639–4644
Arkowitz RA & Abeles RH (1990) Isolation and characterization of a covalent selenocysteine intermediate in the glycine reductase system. J. Am. Chem. Soc. 112: 870–872
Arkowitz RA & Abeles RH (1991) Mechanism of action of clostridial glycine reductase: isolation and characterization of a covalent acetyl enzyme intermediate. Biochemistry 30: 4090–4097
Bader T, Rauschenbach P & Simon H (1982) On a hitherto unknown fermentation path of several amino acids by proteolytic clostridia. FEBS Lett. 140: 67–72
Barker HA (1961) Fermentations of nitrogenous organic compounds. In: Gunsalus IC, Stanier RY (Eds) The bacteria, Vol II (pp 151–207). Academic Press, New York
Barker HA (1981) Amino acid degradation by anaerobic bacteria. Annu. Rev. Biochem. 50: 23–40
Barker HA & Beck JV (1941) The fermentative decomposition of purines byClostridium acidi-urici andClostridium cylindrosporum. J. Biol. Chem. 141: 3–27
Barker HA & Elsden SR (1947) Carbon dioxide utilization in the formation of glycine and acetic acid. J. Biol. Chem. 167: 619–620
Barker HA, Ruben S & Beck JV (1940) Radioactive carbon as an indicator of carbon dioxide reaction. IV. The synthesis of acetic acid from CO2 byClostridium acidi-urici. Proc. Natl. Acad. Sci. (USA) 26: 477–482
Barker HA, Volcani BE & Cardon BP (1948) Tracer experiments on the mechanism of glycine fermentation byDiplococcus glycinophilus. J. Biol. Chem. 173: 803–804
Barnard GF & Akhtar M (1979) Mechanistic and stereochemical studies on the glycine reductase ofClostridium sticklandii. Eur. J. Chem. 99: 593–603
Beuscher HU & Andreesen JR (1984)Eubacterium angustum sp.nov., a Gram-positive, anaerobic, non-sporeforming, obligate purine fermenting organism. Arch. Microbiol. 140: 2–8
Blödorn B (1993) Isolierung eines 63 kDa-Proteins und vergleichende Charakterisierungen mit der Acetat-Kinase ausEubacterium acidaminophilum. Diploma thesis, University of Göttingen
Bourguignon J, Vauclare P, Merand V, Neuburger M & Douce R (1993) Glycine decarboxylasse complex from higher plants. Molecular cloning, tissue distribution and mass spectrometry analyses of the T-protein. Eur. J. Chem. 217: 377–386
Breznak JA (1992) The genusSporomusa. In: Balows A, Trüper HG, Dworkin M, Harder W & Schleifer KH (Eds) The prokaryotes. A handbook on the biology of bacteria: ecophysiology, isolation, identification, applications, Vol 2 (pp 2014–2021). Springer Verlag, New York
Britz ML & Wilkinson RG (1982) Leucine dissimilation to isovaleric and isocaproic acids by cell suspensions of amino acid fermenting anaerobes: the Stickland reaction revisited. Can. J. Microbiol. 28: 291–300
Brookfield DE, Green J, Ali ST, Machado RS & Guest JR (1991) Evidence for two protein-lipoylation activities inEscherichia coli. FEBS Lett. 295: 13–16
Buckel W (1990) Amino acid fermentation: coenzyme B12-dependent and -independent pathways. In: Hauska G, Thauer R (Eds) The molecular basis of bacterial metabolism (pp 21–30). Springer Verlag, Berlin
Cardon BP & Barker HA (1946) Two new amino-acid-fermenting bacteria,Clostridium propionicum andDiplococcus glycinophilus. J. Bacteriol. 52: 629–634
Cardon BP & Barker HA (1947) Amino acid fermentations byClostridium propionicum andDiplococcus glycinophilus. Arch. Biochem. 12: 165–180
Champion AB & Rabinowitz JC (1977) Ferredoxins and formyltetrahydrofolate synthetase: comparative studies withClostridium acidiurici, Clostridium cylindrosporum, and newly isolated anaerobic uric acid-fermenting strains. J. Bacteriol. 132: 1003–1020
Claas JU (1991) Isolierung und Charakterisierung von Ferredoxin und Thioredoxin und deren Einbindung in den Elektronentransport vonEubacterium acidaminophilum. Diploma thesis, University of Göttingen
Cone JE, Del Rio RM & Stadtman TC (1977) Clostridial glycine reductase complex. Purification and characterization of the selenoprotein component. J. Biol. Chem. 252: 5337–5344
Costilow RN (1977) Selenium requirement for the growth ofClostridium sporogenes with glycine as the oxidant in Stickland reaction. J. Bacteriol. 131: 366–368
Csonka LN (1989) Physiological and genetic respones of bacteria to osmotic stress. Microbiol. Rev. 53: 121–147
Dainty RH (1970) Purification and properties of threonine aldolase fromClostridium pasteurianum. Biochem. J. 117: 585–592
Dienes L & Zamacnik PC (1952) Transformation of bacteria into L-forms by amino acids. J. Bacteriol. 64: 770–771
Dietrichs D & Andrcesen JR (1990) Purification and comparative studies on dihydrolipoamide dehydrogenases from anaerobic glycine utilizing bacteriaPeptostreptococcus glycinophilus, Clostridium cylindrosporum, andClostridium sporogenes. J. Bacteriol. 172: 243–251
Dietrichs D, Bahnweg M, Mayer F & Andreesen JR (1991a) Peripheral localization of the dihydrolipoaminde dehydrogenase in the purinolytic anaerobeClostridium cylindrosporum. Arch. Microbiol. 155: 412–414
Dietrichs D, Meyer M, Rieth M & Andreesen JR (1991b) Interaction of selenoprotein Pa and the thioredoxin system, components of the NADPH-dependent reduction of glycine inEubacterium acidaminophilum andClostridium litorale. J. Bacteriol. 173: 5983–5991
Dietrichs D, Meyer M, Schmidt B & Andreesen JR (1990) Purification of NADPH-dependent electron-transferring flavoproteins and N-terminal protein sequence data of dihydrolipoamide dehydrogenases from anaerobic, glycine-utilizing bacteria. J. Bacteriol. 172: 2088–2095
Dürre P & Andreesen JR (1982a) Selenium-dependent growth and glycine fermentation byClostridium purinolyticum. J. Gen. Microbiol. 128: 1457–1466
Dürre P & Andreesen JR (1982b) Separation and quantitation of purines and their anaerobic and aerobic degradation products by high-pressure liquid chromatography. Anal. Biochem. 123: 32–40
Dürre P & Andreesen JR (1983) Purine and glycine metabolism by purinolytic clostridia. J. Bacteriol. 154: 192–199
Dürre P, Spahr R & Andreesen JR (1983) Glycine fermentation via glycine reductase inPeptococcus glycinophilus andPeptococcus magnus. Arch. Microbiol. 134: 127–135
Eklund H, Gleason FK & Holmgren A (1991) Structural and functional relations among thioredoxins of different species. Proteins: Struct. Funct. Genet. 11: 13–28
Elsden SR & Hilton MG (1979) Amino acid utilization patterns in clostridial taxonomy. Arch. Microbiol. 123: 137–141
Fendrich C, Hippe H & Gottschalk G (1990)Clostridium halophilum sp. nov. andC. litorale sp. nov., an obligate halophilic and marine species degrading betaine in the Stickland reaction. Arch. Microbiol. 154: 127–132
Ferris JP, Joshi PC, Edelson EH & Lawless JG (1978) HCN: A plausible source of purines, pyrimidines and amino acids on the primitive earth. J. Mol. Evol 11: 293–311
Finkelstein JD & Martin JJ (1984) Methionine metabolism in mammals. Distribution of homocysteine between competing pathways. J. Biol. Chem. 259: 9508–9513
Foubert EL & Douglas HC (1948) Studies on the anaerobic micrococci. V. Taxonomic considerations. J. Bacteriol. 56: 25–34
Fratini A, Powell BC & Rogers GE (1993) Sequence, expression, and evolutionary conservation of a gene encoding a glycine/tyrosine-rich keratin-associated protein of hair. J. Biol. Chem. 268: 4511–4518
Freudenberg W & Andreesen JR (1989) Purification and partial characterization of the glycine decarboxylase multienzyme complex fromEubacterium acidaminophilum. J. Bacteriol. 171: 2209–2215
Freudenberg W, Dietrichs D, Lebertz H & Andreesen JR (1989a) Isolation of an atypically small lipoamide dehydrogenase involved in the glycine decarboxylase complex fromEubacterium acidaminophilum. J. Bacteriol. 171: 1346–1354
Freudenberg W, Mayer F & Andreesen JR (1989b) Immunocytochemical localization of proteins P1, P2, P3 of glycine decarboxylase, and of the selenoprotein PA of glycine reductase, all involved in anaerobic glycine metabolism ofEubacterium acidaminophilum. Arch. Microbiol. 152: 182–188
Fryer TF & Mead GC (1979) Development of a selective medium for the isolation ofClostridium sporogenes and related organisms. J. Appl. Bacteriol. 47: 425–431
Garcia GE & Stadtman TC (1991) Selenoprotein A component of the glycine reductase complex fromClostridium purinolyticum: nucleotide sequence of the gene shows that selenocysteine is encoded by UGA. J. Bacteriol. 173: 2093–2098
Garcia GE & Stadtman TC (1992)Clostridium sticklandii glycine reductase selenoprotein A gene: cloning, sequencing and expression in Escherichia coli. J. Bacteriol. 174: 7080–7089
Gariboldi RT & Drake HL (1984) Glycine synthase of the purinolytic bacterium,Clostridium acidiurici. Purification of the glycine-CO2 exchange system. J. Biol. Chem. 259: 6085–6089
Gauglitz U (1988) Anaerober mikrobieller Abbau von Kreatin, Kreatinin und N-Methylhydantoin. PhD thesis, University of Göttingen
Giesel H & Simon H (1983) On the occurrence of enoate and 2-oxo-carboxylate reductase in clostridia and some observations on the amino acid fermentation byPeptostreptococcus anaerobius. Arch. Microbiol. 135: 51–57
Giffhorn S (1980) Verwertung von Methanol, Ethanol und Lactat durchClostridium formicoaceticum. Diploma thesis, University of Göttingen
Gleason FK & Holmgren A (1988) Thioredoxin and related proteins in procaryotes. FEMS Microbiol. Rev 54: 271–297
Golovchenko NP, Belokopytov BF & Akimenko VK (1983) Threonine catabolism in the bacteriumClostridium sticklandii. Biochemistry (USSR) 47: 969–974
Gottwald M, Andreesen JR, LeGall J & Ljungdahl LG (1975) Presence of cytochrome and menaquinone inClostridium formicoaceticum andClostridium thermoaceticum. J. Bacteriol. 122: 325–328
Granderath K (1993) Charakterisierung der Formiat-Dehydrogenase und Aldehyd-Dehydrogenase als wolframhaltige Proteine vonEubacterium acidaminophilum. PhD thesis, University of Göttingen
Hammes W, Schleifer KH & Kandler O (1973) Mode of action of glycine on the biosynthesis of peptidoglycan. J. Bacteriol. 116: 1029–1053
Heaton MP, Johnston RB & Thompson TL (1987) Controlled cell lysis and protoplast formation by enhancement of inhibitors of alanine racemase by glycine. Biochem. Biophys Res. Commun. 149: 576–579
Heider J & Böck A (1993) Selenium metabolism in microorganisms. Adv. Microbial. Physiol. 35: 71–133
Heider J, Baron C & Böck A (1992) Coding from a distance: dissection of the mRNA determinants required for the incorporation of selenocysteine into proteins. EMBO J. 11: 3759–3766
Heijthuijsen JHFG & Hansen TA (1989a) Anaerobic degradation of betaine by marineDesulfobacterium strains. Arch. Microbiol. 152: 393–396
Heijthiujsen JHFG & Hansen TA (1989b) Betaine fermentation and oxidation by marineDesulfuromonas strains. Appl. Environ. Microbiol. 55: 965–969
van den Hende C, Oyaert W & Boukert TH (1963) Metabolism of glycine, alanine, valine, leucine and isoleucine by rumen bacteria. Res. Vet. Sci. 4: 382–389
Hermann M, Knerr HJ, Mai N, Groß A & Kaltwasser H (1992) Creatinine and N-methylhydantoin degradation in two newly isolatedClostridium species. Arch. Microbiol. 157: 395–401
Hormann K & Andreesen JR (1989) Reductive cleavage of sarcosine and betaine byEubacterium acidaminophilum via enzyme systems different from glycine reductase. Arch. Microbiol. 153: 50–59
Kamlage B & Blaut M (1993) Isolation of a cytochrome-deficient mutant strain ofSporomusa sphaeroides not capable of oxidizing methyl groups. J. Bacteriol. 175: 3043–3050
Kamlage B, Boelter A & Blaut M (1993) Spectroscopic and potentiometric characterization of cytochromes in twoSporomusa species and their expression during growth on selected substrates. Arch. Microbiol. 159: 189–196
Karlsson JL & Barker HA (1949) Tracer experiments on the mechanism of uric acid decomposition and acetic acid synthesis byClostridium acidi-urici. J. Biol. Chem. 178: 891–902
Kearny JJ & Sagers RD (1972) Formate dehydrogenase fromClostridium acidi-urici. J. Bacteriol. 109: 152–161
Keller B, Sauer N & Lamb CJ (1988) Glycine-rich cell wall proteins in bean: gene structure and association of the protein with the vascular system. EMBO J. 7: 3625–3633
Klein SM & Sagers RD (1966a) Glycine metabolism. I. Properties of the system catalyzing the exchange of bicarbonate with the carboxyl group of glycine inPeptococcus glycinophilus. J. Biol. Chem. 241: 197–207
Klein SM & Sagers RD (1966b) Glycine metabolism. II. Kinetic and optical studies on the glycine decarboxylase system fromPeptococcus glycinophilus. J. Biol. Chem. 241: 206–209
Klein SM & Sagers RD (1967a) Glycine metabolism. III. A flavin-linked dehydrogenase associated with the glycine cleavage system inPeptococcus glycinophilus. J. Biol. Chem. 242: 297–300
Klein SM & Sagers RD (1967b) Glycine metabolism. IV. Effect of borohydride reduction on the pyridoxal phosphate-containing glycine decarboxylase fromPeptococcus glycinophilus. J. Biol. Chem. 242: 301–305
Koo SP & Booth IR (1994) Quantitative analysis of growth stimulation by glycine betaine inSalmonella typhimurium. Microbiology (London) 140: 617–621
Lamark T, Styrvold OB & Strom AR (1992) Efflux of choline and glycine betaine from osmoregulating cells ofEscherichia coli. FEMS Microbiol. Lett 96: 149–154
Lang H, Polster M & Brandsch R (1991) Rat liver dimethylglycine dehydrogenase. Flavinylation of the enzyme in hepatocytes in primary culture and characterization of a cDNA clone. Eur. J. Biochem. 198: 793–799
Lebertz H (1984) Selenabhängiger Glycin-Stoffwechsel in anaeroben Bakterien und vergleichende Untersuchungen zur Glycin-Reduktase und zur Glycin-Decarboxylase. PhD thesis, University of Göttingen
Lebertz H & Andreesen JR (1988) Glycine fermentation byClostridium histolyticum. Arch. Microbiol. 150: 11–14
Lee MJ & Zinder SH (1988) Isolation and characterization of a thermophilic bacterium which oxidzes acetate in syntrophic association with a methanogen and which grows acetogenically on H2-CO2. Appl. Environ. Microbiol. 54: 124–129
Lovitt RW, Morris JG & Kell DB (1987) The growth and nutrition ofClostridium sporogenes NCIB 8053 in defined media. J. Appl. Bacteriol. 62: 71–80
Lübbers M & Andreesen JR (1993) Components of glycine reductase fromEubacterium acidaminophilum. Cloning, sequencing and identification of the genes for thioredoxin reductase, thioredoxin and selenoprotein P A . Eur. J. Biochem. 217: 791–798
Marmelak R & Quastel JH (1953) Amino acid interactions in strict anaerobes (Cl. sporogenes). Biochim. Biophys. Acta 12: 103–120
Marthi B & Lighthart B (1990) Effects of betaine on enumeration of airborne bacteria. Appl. Environ. Microbiol. 56: 1286–1289
Mead GC (1971) The amino acid-fermenting Clostridia. J. Gen. Microbiol. 67: 47–56
Meyer M (1993) Beziehungen des Thioredoxin-Systems zur Glycin-, Sarkosin- und Betain-Reduktase in anaeroben, Aminosäure verwertenden Bakterien. PhD thesis, University of Göttingen
Meyer M, Dietrichs D, Schmidt B & Andreesen JR (1991) Thioredoxin elicits a new dihydrolipoamide dehydrogenase activity by interaction with the electron-transferring flavoprotein inClostridium litorale andEubacterium acidaminophilum. J. Bacteriol. 173: 1509–1513
Molenaar D, Hagting A, Alkema H, Driessen AJM, & Konings WN (1993) Characteristics and osmoregulatory roles of uptake systems for proline and glycine betaine inLactococcus lactis. J. Bacteriol. 175: 5438–5444
Möller B, Hippe H & Gottschalk G (1986) Degradation of various amine compounds by mesophilic clostridia. Arch. Microbiol. 145: 85–90
Möller B, Oßmer R, Howard BH, Gottschalk G & Hippe H (1984)Sporomusa, a new genus of gram-negative anaerobic bacteria includingSporomusa sphaeroides spec. nov. andSporomusa ovata spec. nov. Arch. Microbiol. 139: 388–396
Müller E, Fahlbusch K, Walther R & Gottschalk G (1981) Formation of N, N-dimethylglycine, acetic acid, and butyric acid from betaine byEubacterium limosum. Appl. Environ. Microbiol. 42: 439–445
Murao S, Hinode Y, Matsumura E, Numata A, Kawai K, Ohishi H, Jin H, Oyama H & Shin T (1992) A novel laccase inhibitor, N-hydroxyglycine, produced byPenicillium citrinum YH-31. Biosci. Biotech. Biochem. 56: 987–988
Nagase M & Matsuo T (1982) Interactions between amino-acid-degrading bacteria and methanogenic bacteria in anaerobic digestion. Biotechnol. Bioeng. 24: 2227–2239
Naumann E, Hippe H & Gottschalk G (1983) Betaine: new oxidant in the Stickland reaction and methanogenesis from betaine and L-alanine by aClostridium sporogenes — Methanosarcinabarkeri coculture. Appl. Environ. Microbiol. 45: 474–483
Newmann EB, D'Ari & Lin RT (1992) The leuchine-Lrp regulon inEscherichia coli: a global response in search of a raison d'etre. Cell 68: 617–619
Nisman B (1954) The Stickland reaction. Bacteriol. Rev. 18: 16–42
Okamura-Ikeda K, Ohmura Y, Fujiwara K & Motakawa Y (1993) Cloning and nucleotide sequence of the gcv operon encoding theEscherichia coli glycine-cleavage system. Eur. J. Biochem. 216: 539–548
Oppermann FB & Steinbüchel (1994) Identification and molecular characterization of the aco genes encoding thePelobacter carbinolicus acetoin dehydrogenase enzyme system. J. Bacteriol. 176: 469–485
Oren A (1990) Formation and breakdown of glycine betaine and trimethylamine in hypersaaline environments. Antonie van Leeuwenhoek 58: 291–298
Phelps TJ & Zeikus JG (1984) Influence of pH on terminal carbon metabolism in anoxic sediments from a mildly acidic lake. Appl. Environ. Microbiol. 48: 1088–1095
Pinsent J (1954) The need of selenite and molybdate in the formation of formic dehydrogenase by members of the coli-aerogenase group of bacteria. Biochem. J. 57: 10–16
van Poelje PD & Snell EE (1990) Pyruvoyl-dependent enzymes. Annu. Rev. Biochem. 59: 29–59
Porter DH, Cook RJ & Wagner C (1985) Enzymatic properties of dimethylglycine dehydrogenase and sarcosine dehydrogenase from rat liver. Arch. Biochem. Biophys 243: 396–407
Rhodes D & Hanson AD (1993) Quarternary ammonium and tertiary sulfonium compounds in higher plants. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 44: 357–384
Richarme G (1989) Purification of a new dihydrolipoamide dehydrogenase fromEscherichia coli. J. Bacteriol. 171: 6580–6585
Rieth M (1987) Untersuchungen zur selenabhängigen Glycinreduktase ausEubacterium acidaminophilum. PhD thesis, University of Göttingen
Robinson JR, Klein SM & Sagers RD (1973) Glycine metabolism. Lipoic acid as the prosthetic group in the electron transfer protein P2 fromPeptococcus glycinophilus. Biol. Chem. 248: 5319–5323
Schiefer-Ullrich H & Andreesen JR (1985)Peptostreptococcus barnesae sp. nov., a Gram-positive, anaerobic, obligately purine utilizing coccus from chicken feces. Arch. Microbiol. 143: 26–31
Schiefer-Ullrich H, Wagner R, Dürre P & Andreesen JR (1984) Comparative studies on physiology and taxonomy of obligately purinolytic clostridia. Arch. Microbiol. 138: 345–353
Schirch V & Strong WB (1989) Interaction of folylpolyglutamates with enzymes in one-carbon metabolism. Arch. Biochem. Biophys. 269: 371–380
Schleicher A (1990) Anaerober Abbau von Kreatinin und Kreatin unter Beteiligung von Reduktase-Reaktionen. Diploma thesis, University of Göttingen
Schräder T & Andreesen JR (1992) Purification and characterization of protein Pc, a component of glycine reductase fromEubacterium acidaminophilum. Eur. J. Biochem. 206: 79–85
Schwartz AC, Quecke W & Brenschede (1979) Inhibition by glycine of the catabolic reduction of proline inClostridium sticklandii: evidence on the regulation of amino acid reduction. Z. Allg. Microbiol. 19: 211–220
Seto B (1980) The Stickland reaction. In: Knowles CJ (Ed) Diversity in bacterial respiratory systems, Vol II (pp 49–64). CRC Press, Boca Raton
Sliwkowski MX & Stadtman TC (1987) Purification and immunological studies of selenoprotein A of the clostridial glycine reductase complex. J. Biol. Chem. 262: 4899–4904
Sliwkowski MX & Stadtman TC (1988a) Selenoprotein A of the clostridial glycine reductase complex: purification and amino acid sequence of the selenocysteine-containing peptide. Proc. Natl. Acad. Sci. (USA) 85: 368–371
Sliwkowski MX & Stadtman TC (1988b) Selenium-dependent glycine reductase: differences in physicochemical properties and biological activities of selenoprotein A components isolated fromClostridium sticklandii andClostridium purinolyticum. Biofactor. 1: 293–296
Stadtman TC (1962) Studies on the enzymic reduction of amino acids. V. coupling of a DPNH-generating system to glycine reduction. Arch. Biochem. Biophys. 99: 36–44
Stadtman TC (1965) Electron transport proteins ofClostridium sticklandii. In: San Pietro A (Ed) Non-heme-iron proteins, role in energy conservation (pp 439–445) Antioch Press, Yellow Springs
Stadtman TC (1966) Glycine reduction to acetate and ammonia: identification of ferredoxin and another low molecular weight acidic protein as components of the reductase system. Arch. Biochem. Biophys. 113: 9–19
Stadtman TC (1978) Selenium-dependent clostridial glycine reductase. Meth. Enzymol. 53: 373–382
Stadtman TC (1989) Clostridial glycine reductase Protein C, the acetyl group acceptor catalyzes the arsenate-dependent decomposition of acetyl phosphate. Proc. Natl. Acad. Sci. (USA) 86: 7853–7856
Stadtman TC & Davis JN (1991) Glycine reductase protein C. Properties and characterization of its role in the reductive cleavage of Se-carboxymethylselenoprotein A. J. Biol. Chem. 266: 22147–22153
Stadtman TC & Elliott P (1956) A new ATP-forming reaction: the reductive deamination of glycine. J. Am. Chem. Soc. 78: 2020–2021
Stadtman TC, Elliott P & Tiemann L (1958) Studies on the enzymic reduction of amino acids. III. Phosphate esterification coupled with glycine reduction. J. Biol. Chem. 231: 961–973
Stadtman TC & White FH (1954) Tracer studies on ornithine, lysine, and formate metabolism in an amino acid fermenting Clostridium. J. Bacteriol. 67: 651–657
Stams AJM & Hansen TA (1984) Fermentation of glutamate and other compounds byAcidaminobacter hydrogenoformans gen. nov. sp. nov., an obligate anaerobe isolated from black mud. Studies with pure cultures and mixed cultures with sulfate-reducing and methanogenic bacteria. Arch. Microbiol. 137: 329–337
Stams AJM, Hansen TA & Skyring GW (1985) Utilization of amino acids as energy substrates by two marineDesulfovibrio strains. FEMS Microbiol. Ecol. 31: 11–15
Steiert JG, Rolfes RJ, Zalkin H & Stauffer GV (1990a) Regulation of theEscherichia coli gly A gene by the pur R gene product. J. Bacteriol. 172: 3799–3803
Steiert PS, Stauffer LT & Stauffer GV (1990b) The lpd gene product functions as the L protein in theEscherichia coli glycine cleavage enzyme system. J. Bacteriol. 172: 6142–6144
Stickland LH (1934) The chemical reaction by whichCl. sporogenes obtains its energy. Biochem. J. 28: 1746–1759
Stickland LH (1935a) Studies in the metabolism of the strict anaerobes (GenusClostridium) II. The reduction of proline byCl. sporogenes. Biochem. J. 29: 288–290
Stickland LH (1935b) Studies in the metabolism of the strict anaerobes (GenusClostridium) III. The oxidation of alanine byCl. sporogenes. IV. The reduction of glycine byCl. sporogenes. Biochem. J. 29: 889–898
Szulmajster J (1958) Bacterial fermentation of creatinine. I. Isolation of N-methyl-hydantoin. J. Bacteriol. 75: 633–639
Szulmajster J (1960) Le carbamyl-phosphate, intermediaire dans la degradation de la creatinine per des extraits enzymatique d'Eubacterium sarcosinogenum. Biochim. Biophys. Acta 44: 173–175
Szulmajster J & Gardiner RC (1960) Enzymatic formation of polyphosphate in an anaerobic bacterium. Biochim. Biophys. Acta 39: 165–167
Szulmajster J & Kaiser P (1960) Etude d'une nouvelle espece anaerobie:Eubacterium sarcosinogenum nov. sp. Ann. Inst. Pasteur. 98: 774–777
Tanaka H & Stadtman TC (1979) Selenium-dependent clostridial glycine reductase. Purification and characterization of the two membrane-associated protein components. J. Biol. Chem. 254: 447–452
Tanaka K & Pfennig N (1988) Fermentation of 2-methoxyethanol byAcetobacterium malicum sp. nov. and Pelobacter venetianus. Arch. Microbiol. 149: 181–187
Thauer, RK, Jungermann K & Decker K (1977) Energy conservation in chemotro-phic anaerobic bacteria. Bacteriol. Rev. 41: 100–180
Turner DC & Stadtman TC (1973) Purification of protein components of the clostridial glycine reductase system and characterization of protein A as a selenoprotein. Arch. Biochem. Biophys. 154: 366–381
Tziaka C (1987) Untersuchungen zur Charakterisierung von Stämmen der GattungenPeptococcus undPeptostreptococcus. PhD thesis, University of Göttingen
Uhde A (1990) Wachstumsphysiologische Untersuchungen zum Abbau von Aminosäuren und mögliche Funktion eines elektronentransferierenden Flavoproteins beiClostridium sticklandii. Diploma thesis, University of Göttingen
Vanden Boom TJ, Reed KE & Cronan JE (1991) Lipoic acid metabolism inEscherichia coli: isolation of null mutants defective in lipoic acid biosynthesis, molecular cloning and characterization of the E. coli lip locus, and identification of the lipolyated protein of the glycine cleavage system. J. Bacteriol. 173: 6411–6420
Venugopalan V (1980) Influence of growth conditions on glycine reductase ofClostridium sporogenes. J. Bacteriol. 141: 386–388
Vogels GD & van der Drift C (1976) Degradation of purines and pyrimidines by microorganisms. Bacteriol. Rev. 40: 403–468
Waber JL & Wood HG (1979) Mechanism of acetate synthesis from CO2 byClostridium acidi-urici. J. Bacteriol. 140: 468–478
Wagner M (1994) Isolierung und Charakterisierung der Threonin-Dehydrogenase ausClostridium sticklandii. Diploma thesis, University of Göttingen
Wagner R & Andreesen JR (1987) Accumulation and incorporation of185W-tungsten into proteins ofClostridium acidiurici andClostridium cylindrosporum. Arch. Microbiol. 147: 295–299
Wagner R, Cammack R & Andreesen JR (1984) Purification and characterization of xanthine dehydrogenase fromClostridium acidiurici grown in the presence of selenium. Biochim. Biophys. Acta 791: 63–74
Wang Q, Wu J, Friedberg D, Plakto J & Calvo JM (1994) Regulation of theEscherichia coli lrp gene. J. Bacteriol. 176: 1831–1839
Williams CH (1992) Lipoamide dehydrogenase, glutathione reductase, thioredoxin reductase, and mercuric ion reductase — a family of flavoenzyme transhydroge-nases. In: Müller F (Ed) Chemistry and biochemistry of flavoenzymes, Vol 3 (pp 121–211). CRC Press, Boca Raton
Wilson RL & Stauffer GV (1994) DNA sequence and characterization of GcvA, a LysR family regulatory protein for theEscherichia coli glycine cleavage enzyme system. J. Bacteriol. 176: 2862–2868
Wilson RL, Stauffer LT & Stauffer GV (1993a) Roles of the GevA and PurR proteins in negative regulation of theEscherichia coli glycine cleavage enzyme system. J. Bacteriol. 175: 5129–5134
Wilson RL, Steiert PS & Stauffer GV (1993b) Positive regulation of theEscherichia coli glycine cleavage enzyme system. J. Bacteriol. 175: 902–904
Winter J, Schindler F & Wildenauer FX (1987) Fermentation of alanine and glycine by pure and syntrophic cultures ofClostridium sporogenes. FEMS Microbiol. Ecol. 45: 153–161
Woods DD (1936) Studies in the metabolism of the strict anaerobes (GenusClostridium). V. Further experiments on the coupled reactions between pairs of amino-acids induced byCl. sporogenes. Biochem. J. 30: 1934–1946
Wood HG & Ljungdahl LG (1991) Autotrophic character of the acetogenic bacteria. In: Shively JM, Barton LL (Eds) Variations in autotrophic life (pp 201–250). Academic Press, London
Wright DE & Hungate RE (1967a) Amino acid concentrations in rumen fluid. Appl. Microbiol. 15: 148–151
Wright DE & Hungate RE (1967b) Metabolism of glycine by rumen microorganisms. Appl. Microbiol. 15: 152–157
Zhilina TN & Zavarzin GA (1990) Extremely halophilic, methylotrophic, anaerobic bacteria. FEMS Microbiol. Rev 87: 315–321
Zindel U, Freudenberg W, Rieth M, Andreesen JR, Schnell J & Widdel F (1988)Eubacterium acidaminophilum sp. nov., a versatile amino acid-degrading anaerobe producing or utilizing H2 or formate. Description and enzymatic studies. Arch. Microbiol. 150: 254–266
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Andreesen, J.R. Glycine metabolism in anaerobes. Antonie van Leeuwenhoek 66, 223–237 (1994). https://doi.org/10.1007/BF00871641
Issue Date:
DOI: https://doi.org/10.1007/BF00871641