Abstract
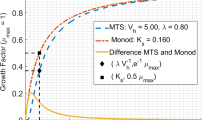
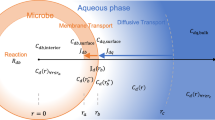
Traditional concepts of nutrient uptake and growth kinetics as linked by cell yield are presented. Phenomena affecting the kinetics are examined along with a discussion of those which lead to ambiguity. Concepts of flux control are presented to help understand the distribution of material along metabolic pathways. Specific affinity is described to relate nutrient accumulation rates to transporter density. It is shown to be a primary kinetic constant and the best available index of nutrient collection ability. As an aid to understanding, specific affinity is reexpressed in terms of membrane permeability. Formulations of nutrient transport rate as a function of cellular composition, particularly transporter and enzyme content and known as janusian kinetics, are described as an improvement to specific affinity theory. Procedures for quantified unidirectional fluxes are reviewed to identify the difference between gross and net transport rates of substrate. Collision frequency theory is used to show that in addition to total biomass, cell size and transporter density should also be included in rate equations describing microbial growth. Theory diversity suggests that one reason for microbial metabolic is that the likelihood of additional collisions of substrate molecules with a cell surface, after an initial collision, requires only a sparse distribution of transporter sites for maximal rate, leaving room for additional transporters able to collect other substrate types.
Similar content being viewed by others
References
Akagi Y & Taga N (1980) Uptake of D-glucose and L-proline by oligotrophic and heterotrophic marine bacteria. Can. J. Microbiol. 26: 454–459
Albertson H, Nystrĵm T & Kjelleberg S (1990) Starvation induced modulations in binding protein-dependent glucose transport by the marineVibrio sp. S14. FEMS Microbiol. Lett. 70: 205–210
Abbott AJ & Nelsetuen GL (1988) The collisional limit: an important consideration for membrane-associated enzymes. Fed. Am. Soc. Exp. Biol. J. 2: 2858–2866
Azam F & RE Hodson (1981) Multiphasic kinetics for D-glucose uptake by assemblages of natural marine bacteria. Mar. Ecol. Prog. Ser. 6: 213–222
Berg, HC & Purcell EM (1977) Physics of chemoreception. Biophys. J. 20: 193–215
Button DK (1991) Biochemical basis for whole-cell uptake kinetics: specific affinity, oligotrophic capacity, and the meaning of the Michaelis constant. Appl. Environ. Microbiol. 57: 2033–2038
Button DK & Kinney PJ (1980) Unidirectional flux determination during nutrient limited microbial growth by the isotope relaxation rate induced in continuous culture. In: Sikyta B. Fencel Z & Polacek V (Eds) Continuous Cultivation of Microorganisms. Proceedings of the 7th symposium (pp 269–278)
Button DK & Robertson BR (1987) Toluene induction and uptake kinetics and their inclusion into the specific-affinity equation for describing rates of hydrocarbon metabolism. Appl. Environ. Microbiol. 53: 2193–2205
Button DK, Dunker SS & Morse ML (1973) Continuous culture ofRhodotorula rubra: kinetics of phosphate-arsenate uptake, inhibition and phosphate limited growth. J. Bacteriol. 3: 599–611
Button DK, Robertson BR, McIntosh D & Jüttner F (1992) Interactions between marine bacteria and dissolved-phase and beached hydrocarbons after theExxon Valdez oil spill. Appl. Environ. Microbiol. 58: 243–25
Chesbro W (1988) The domains of slow bacterial growth. Can. J. Microbiol. 34: 427–435
Chisholm SW (1992) Phytoplankton size. In: Falkowaski PG & Woodhead AD (Eds) Primary Productivity and Biochemical Cycles in the Sea. Plenum, New York
Cleland WW (1975) Partition analysis and the concept of net rate constants as tools in enzyme kinetics. Biochemistry 14: 3220–3224
Cleland WW (1987) The use of isotope effects in the detailed analysis of catalytic mechanisms of enzymes. Bioorg. Chem. 15: 283–302
Droop MR (1968) Vitamin B12 and marine ecology. IV. The kinetics of uptake, growth and inhibition inMonochrysis lutheri. J. Mar. Biol. Assoc. U.K. 48: 689–733
Egli T, Lendenmann U & Snozzi M (1992) Kinetics of microbial growth with mixtures of carbon sources. Antonie van Leeuwenhoek 63: 289–298 (this issue)
Kell DB & Westerhoff HV (1986) Metabolic control theory: its role in microbiology and biotechnology. FEMS Microbiol. Rev. 39: 305–320
Raw RAT, Robertson BR & Button DK (1976) On describing microbial growth kinetics from continuous culture data. Some general considerations, observations and concepts. Microb. Ecol. 2: 261–283
Martinez MB, Schendel FJ, Flickiger MC & Nelsetuen GL (1992) Kinetic properties of enzyme regulationin vivo: Alkaline phosphatase of theE. coli periplasm. Biochemistry 31: 11500–11509
Michaelis L & Menten MM (1913) Die Kinetik der Invertinwirkung. Biochem. Z. 49: 333–369
Monod J (1950) Technique de culture continue. Theorie et applications. Ann. Inst. Pasteur 79: 390–410
Neal J (1972) Analysis of Michaelis kinetics for two independent, saturable membrane transport functions. J. Theor. Biol. 35: 113–118
Newsholme EA & Crabtree B (1981) Flux-generating and regulatory steps in metabolic control. Trends Biochem. Sci. 6: 53–56
Olson RJ, Vaulot D & Chisholm SW (1977) Marine phytoplankton distributions measured using shipboard flow cytometry. Deep-Sea Res. 32: 1273–1280
Robertson BR & Button DK (1979) The Phosphate-limited continuous culture ofRhodotorula rubra: kinetics of transport, leakage and growth. J. Bacteriol. 138: 884–895
Robertson BR & Button DK (1987) Toluene induction and uptake kinetics and their inclusion in the specific-affinity relationship for describing rates of hydrocarbon metabolism. Appl. Environ. Microbiol. 53: 2193–2205
Schmidt TM, DeLong EF & Pace NR (1991). Analysis of a marine picoplankton community by 16S rNA gene cloning and sequencing. J. Bacteriol. 173: 4371–4378
Sen K, Hellman J & Nikaido H (1980) Porin channels in intact cells ofEscherichia coli are not affected by Donnan potentials across the outer membrane. J. Biol. Chem. 263: 1182–1187
Van Dam K, Postma P, Ruijter G, Ruthers M, van der Vlag J & Westerhoff HV (1993) Control and regulation of metabolic fluxes in microbes by substrates and enzymes. Antonie van Leeuwenhoek (this issue)
Wright RT & Hobbie JE. (1965) The uptake of organic solutes in lake water. Limnol. Oceanogr. 10: 22–28
Zimmermann RC, Kremer JN & Dugdale RC (1987). Acceleration of nutrient uptake by phytoplankton in a coastal upwelling ecosystem: A modeling analysis. Limnol. Oceanogr. 32: 359–367
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Button, D.K. Nutrient-limited microbial growth kinetics: overview and recent advances. Antonie van Leeuwenhoek 63, 225–235 (1993). https://doi.org/10.1007/BF00871220
Issue Date:
DOI: https://doi.org/10.1007/BF00871220