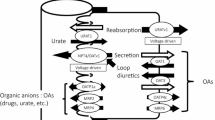
Abstract
This article reviews recent advances in the mechanisms of renal amino acid transport. Renal amino acid transport is necessary to efficiently reclaim approximately 450 mmol amino acids from the glomerular ultrafiltrate each day in man. In general, individual amino acids are transported across the epithelial membrane of the proximal tubule by a sodium (Na+) dependent mechanism. This cotransport process utilizes the energy of the Na+ gradient to enter the cell. The amino acid then exits the basolateral surface and Na+ is pumped out by the Na+−K+-ATPase located in the basolateral membrane. In addition to the cellular accumulation of amino acids across the luminal membrane, these compounds may be taken up by the cell from the basolateral surface. Most amino acids are transported both individually and in a series of seven group specific processes. Human disorders of amino acid transport have been described for six to the seven transport systems. The process of ontogeny of amino acid accumulation by the proximal tubule is a complex one and will be further discussed in this review. A number of factors including pH, ion dependency, electrogenicity of transport process, as well as a variety of hormonal factors, may contribute to the regulation of amino acid transport. Gene expression of several amino acid transporters has been successfully performed using the oocyte of the frogXenopus laevis. Using this system, a number of transporters have been cloned. Such a strategy will permit the cloning of virtually all transporter molecules, and thus we can anticipate the elucidation of the structure of the transporters. However, for a comprehensive understanding of cytoskeletal interactions protein phosphorylation and phospholipid domains and their linkage to the primary structure of the transporter need to be studied. The future for research in this area is indeed a bright one.
Similar content being viewed by others
References
Trachman H (1991) Cell volume regulation: a review of cerebral adaptive mechanisms and clinical treatment of osmolal disturbances. Pediatr Nephrol 5: 743–750
Zelikovic I, Chesney RW (1989) Sodium coupled amino acid transport in renal tubule. Kidney Int 36: 351–359
Silbernagl S (1988) The renal handling of amino acids and oligopeptides. Physiol Rev 68: 911–1007
Silbernagl S (1992) Tubular transport of amino acids and small peptides. In: Windhanger EE (ed) Handbook of physiology — renal physiology. Oxford University Press, New York, pp 1937–1976
Emanual JR, Garetz S, Stone L, Levenson R (1987) Differential expression of Na+, K+-ATPase α- and β-subunit mRNAs in rat tissues and cell lines. Proc Natl Acad Sci USA 84: 9030–9034
Sardet C, Counillon L, Franchi A, Pouyssegur J (1990) Growth factors induce phosphorylation of the Na+/H+ antiporter, a glycoprotein of 100 kD. Science 247: 723–725
Silbernagl S, Ganapathy V, Leibach FH (1987) H+-gradient-driven dipeptide reabsorption in the proximal tubule of rat kidney. Studies in vivo and in vitro. Am J Physiol 253: F448-F457
Barfuss DW, Mays JM, Schafer JA (1980) Peritubular uptake and transepithelial transport of glycine in isolated proximal tubules. Am J Physiol 238: F324-F333
Chesney RW (1993) Membrane transport systems: iminoglycinuria. In: Scriver CR, Beaudet AL, Sly W, Valle D (eds) The metabolic basis of inherited diseases, 7th edn. McGraw-Hill, New York (in press)
Zelikovic I, Stejskal-Lorenz E, Lohstroh P, Budreau A, Chesney RW (1989) Anion dependence of taurine transport by rat renal brushborder membrane vesicles. Am J Physiol 256: F646-F655
Schneider EG, Sacktor B (1980) Sodium gradient-dependentl-glutamate transport in renal brush-border membrane vesicles. Evidence for an electroneutral mechanism. J Biol Chem 255: 7650–7656
Kinne RKH (1988) Polarity, diversity, and plasticity in proximal tubule transport systems. Pediatr Nephrol 2: 477–484
Silbernagl S, Völkl H (1983) Molecular specificity of the tubular reabsorption of “acidic” amino acids. A continuous microperfusion study in rat kidney in vivo. Pflugers Arch 396: 225–230
Kinne RKH (1991) Selectivity and direction: plasma membranes in renal transport. Am J Physiol 260: F153-F162
Jessen H, Sheikh MI (1991) Renal transport of taurine in luminal membrane vesicles from rabbit proximal tubule. Biochim Biophys Acta 1064: 189–198
Segal S, Smith I (1969) Delineation of cystine transport systems in rat kidney cortex by developmental patterns. Proc Natl Acad Sci USA 63: 926–933
Christensen HN (1985) On the strategy of kinetic discrimination of amino acid transport systems. J Membr Biol 84: 97–103
Friedman AL, Chesney RW (1988) Isolated renal tubular disorders. In: Schrier RW, Gottschalk CW (eds) Diseases of the kidne 4th edn. Little, Brown, Boston, pp 663–688
Scriver CR, Beaudet A, Valle D, Sly W (1990) Inherited basis of metabolic disorders, 6th edn. McGraw Hill, New York, pp 2463–2481
Zelikovic I, Chesney RW (1989) Development of renal amino acid transport systems. Semin Nephrol 9: 49–55
Medow MS, Foreman JW, Bovee KC, Segal S (1982) Developmental changes of glycine transport in the dog. Biochim Biophys Acta 693: 85–92
Barlocher KE, Scriver CR, Mohyuddin F (1970) Ontogeny of iminoglycine transport in mammalian kidney. Proc Natl Acad Sci USA 65: 1009–1016
Baerlocher KE, Scriver CR, Mohyuddin F (1971) The ontogeny of amino acid transport in rat kidney. 1. Effect on distribution ratios and intracellular metabolism of proline and glycine. Biochim Biophys Acta 249: 353–363
Webber WA, Cairns JS (1968) A comparison of the amino acid concentrating ability of the kidney cortex of newborn and mature rats. Can J Physiol Pharmacol 46: 165–169
Foreman JW, Medow MS, Bovee KC, Segal S (1986) Developmental aspects of cystine transport in the dog. Pediatr Res 20: 593–597
Segal S, Smith I (1969) Delineation of separate transport systems in rat kidney cortex forl-lysine andl-cystine by developmental patterns. Biochem Biophys Res Commun 35: 771–777
Medow MS, Roth KS, Godman DR (1986) Developmental aspects of proline transport in rat renal brush border memberanes. Proc Natl Acad Sci USA 83: 7561–7564
Goldmann DR, Roth KS, Langfitt TW, Segal S (1979)l-Proline transport by newborn rat kidney brush border membrane vesicles. Biochem J 178: 253–256
Zelikovic I, Stejskal-Lorenz E, Lohstroh P, Budreau A, Chesney RW (1991) Developmental maturation of Na+/H+ exchange in rat renal tubular brush border membrane. Am J Physiol 261: F1017-F1025
Hwang SM, Serahan MA, Roth KS, Segal S (1983)l-proline transport by isolated renal tubules from newborn and adult rats. Pediatr Res 17: 42–46
Medow MS, Segal S (1987) Age related changes in fluidity of rat renal brush border membrane vesicles. Biochem Biophys Res Commun 142: 849–856
Chesney RW, Gusowski N, Zelikovic I, Padilla M (1986) Developmental aspects of renal β-amino acid transport. V. Brush border membrane transport in nursing animals — effect of age and diet. Pediatr Res 20: 890–895
Chesney RW, Jax DK (1979) Developmental aspects of renal β-amino acid transport. I. Ontogeny of taurine reabsorption and accumulation in rat renal cortex. Pediatr Res 13: 854–860
Chesney RW, Jax DK (1979) Developmental aspects of renal β-amino acid transport. II. Ontogeny of uptake and efflux processes and effect of anoxia. Pediatr Res 13: 861–867
Zelikovic I, Chesney RW, Ahlfors EC (1990) Very low birth weight (VLBW) infants receiving prolonged total parenteral nutrition (TPN) are taurine depleted because of renal immaturity. J Pediatr 116: 301–306
Helms RA, Chrisensen ML, Storm MC, Chesney RW. Sulfur amino acid metabolism in preterm infants on parenteral nutrition. J Pediatr
Chesney RW, Zelikovic I, Jones DP, Budreau A, Jolly K (1990) The renal transport of taurine and regulation of renal sodium-chloride-dependent transporter activity. Pediatr Nephrol 4: 399–407
Zelikovic I, Budreau A, Randle A, Chesney RW (1993) Cl- and membrane potential dependence of Na+ coupled amino acid transport across the rat renal tubular brush border membrane. Biochim Biophys Acta, in press.
Chesney RW, Zelikovic I, Budreau AM, Randle D (1991) Chloride and membrane potential dependence of sodium ion-proline symport. J Am Soc Nephrol 2: 885–893
Roigaard-Petersen H, Jessen H, Mollerup S, Jorgensen KE, Jacobsen C, Sheikh MI (1990) Proton gradient-dependent renal transport of glycine: evidence from vesicle studies. Am J Physiol 258: F388-F396
Chesney RW, Gusowski N, Dabbagh S (1985) Renal cortex taurine content regulates the adaptive response to altered dietary intake of sulfur amino acids. J Clin Invest 76: 2213–2222
Jones DP, Miller LA, Chesney RW (1990) Adaptive regulation of taurine transport in two continuous renal epithelial cell lines. Kidney Int 38: 219–226
Chesney RW, Gusowski N, Friedman AL (1983) Renal adaptation to altered amino acid intake occurs at the luminal brush border membrane. Kidney Int 24: 588–594
Jones DP, Miller LA, Dowling C, Chesney RW (1991) Regulation of taurine transporter activity in LLC-PK cells: role of protein synthesis and protein kinase C activation. J Am Soc Nephrol 2: 1021–1029
Chesney RW, Jolly K, Zelikovic I, Iwakashi C, Lohstroh P (1989) Increased Na+-taurine symporter in rat renal brush border membranes: performed or newly synthesized protcin. FASEB J 3: 2081–2085
Jones DP, Miller LA, Chesney RW (1992) Polarity of transporter regulation: apical hypertonicity increases basolateral taurine transport in MDCK cells. Pediatr Res 31: 335A
Uchida S, Kwon HM, Preston AS, Handler JS (1991) Expression of madin-darby canine kidney cell Na+- and Cl-dependent taurine transporter inXenopus laevis oocytes. J Biol Chem 266: 9605–9609
Jones DP, Miller LA, Budreau A, Chesney RW (1992) Characteristics of taurine transport in cultured renal epithelial cell lines: asymmetric polarity of proximal and distal cell lines. Lombardini JB et al. (eds) Taurine. Plenum, New York, pp 405–411
Sanchez OR, Pasantes-Morales H, Lazaro A, Cereijido M (1991) Osmolarity-sensitive release of free amino acids from cultured renal cells. J Membr Biol 121: 1–9
Terreros D, Kanli H, Fugelli K (1992) Taurine efflux as part of cell volume regulatory decreases in proximal renal tubules. J Am Soc Nephrol 3: 836
Chesney RW, Budreau AM (1993) Efflux of taurine from renal brush border membrane vesicles: is it adaptively regulated? Pediatr Nephrol 7: 35–40
Zelikovic I, Przekwas J (1992) Ca++-dependent protein kinases in the rat kidney during development. J Am Soc Nephrol 3: 513
Zelikovic I, Przekwas J (1993) The role of protein phosphorylation in renal amino acid transport. Pediatr Nephrol (in press)
Grose JH, Scriver CR (1968) Parathyroid hormone dependent phosphaturia and aminoaciduria. Am J Physiol 214: 370–378
Dabbagh S, Chesney RW, Gusowski N, Matthews MC Padilla M, Theissen M, Slatopolsky E (1989) Aminoaciduria of vitamin D deficiency is independent of PTH levels and urinary cyclic AMP. Miner electrolyte Metab 15: 221–232
Dabbagh S, Gusowski N, Chesney RW, Falsetti G, Ellis M, Ellis D (1989) Cyclic AMP does not alter taurine accumulation by rat renal brush border membrane vesicles. Biochem Med Metab Biol 42: 132–145
Dabbagh S, El-Baba M, Somnath M (1992) Effect of phosphate (P) depletion and vitamin D deficiency on the stoichiometry of the taurine symport. Proceedings of the Ninth Congress of the International Pediatric Nephrology Association, Jerusalem, Israel. Pediatric Nephrol 6: C67
Hediger MA, Ikeda T, Coady M, Gundersen CB, Wright EM (1987) Expression of size-selected mRNA encoding the intestinal Na/glucose cotransporter inXenopus laevis oocytes. Proc Natl Acad Sci USA 84: 2634–2637
Hediger MA, Coady JM, Ikeda TS, Wright EM (1987) Expression cloning and cDNA squencing of the Na+/glucose co-transporter. Nature 330: 379–381
Hediger MA, Turk E, Wright EM (1989) Homology of the human intestinal Na+/glucose andEscherichia coli Na+/proline cotransporters. Proc Natl Acad Sci USA 86: 5748–5752
Weber W-M, Püschel B, Steffgen J, Koepsell H, Schwartz W (1991) Comparison of a Na+/D-glucose cotransporter from rat intestine expressed in oocytes ofXenopus laevis with the endogenous cotransporter. Biochim Biophys Acta 1063: 73–80
Kim JW, Closs EI, Albritton LM, Cunningham JM (1991) Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature 352: 725–728
Albritton LM, Tseng L, Scadden D, Cunningham JM (1989) A putative murine ecotropic retrovirus receptor gene encodes a multiple membrane-spanning protein and confers susceptibility to virus infection. Cell 57: 659–666
McNamara PD, Rea CT, Segal S (1991) Expression of rat jejunal cystine carrier inXenopus oocytes. J Biol Chem 266: 986–989
Uchida S, Kwon HM, Yamauchi A, Preston AS, Marumo F, Handler JS (1992) Molecular cloning of the cDNA for an MDCK cell Na+- and Cl-dependent taurine transporter that is regulated by hypertonicity. Proc Natl Acad Sci USA 89: 8230–8234
Law RO (1991) Amino acids as volume-regulatory osmolytes in mammalian cells. Comp Biochem Physiol [A] 99A: 263–277
Burg MB, Garcia-Perez A (1992) How tonicity regulates gene expression. J Am Soc Nephrol 3: 121–127
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chesney, R.W., Jones, D. & Zelikovic, I. Renal amino acid transport: cellular and molecular events from clearance studies to frog eggs. Pediatr Nephrol 7, 574–584 (1993). https://doi.org/10.1007/BF00852553
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00852553