Abstract
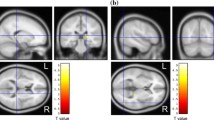
Dementia with Lewy bodies (DLB) can at present only be diagnosed with certainty by neuropathological examination. Diagnosis during life remains at best probable, based on the presence of symptoms known from autopsy studies to be frequently associated with DLB. The greatest practical clinical problem lies in distinguishing DLB arid Alzheimer's disease (AD). In DLB there is a considerable degeneration of nigral neurones with depletion of striatal dopamine. In contrast, AD is not associated with significant changes in dopamine metabolism. Iodine-123 iodobenzamide single-photon emission tomography (IBZM-SPET) measures post-synaptic dopamine D2 neuroreceptor availability in the corpus striatum, but is nevertheless a method for assessing the integrity of the nigrostriatal dopaminergic pathway. Sixteen clinically diagnosed DLB patients, 15 normal controls arid 13 AD patients underwent IBZM-SPET. All subjects were scanned 1.5–2 h after intravenous injection of 185 MBq of123I-IBZM. Circular regions of iriterest were employed to calculate radioactivity ratios in each hemisphere as follows: caudate nucleus/frontal cortex, putamen/frontal cortex arid caudate nucleus/putamen. The DLB patients had significantly lower left caudate/putamen ratios (95% confidence intervals: DLB 0.893–0.965, AD 0.972–1.175, controls 1.031–1.168) than either controls or AD patients, and significantly lower right caudate/putamen ratios (95% confidence intervals: DLB 0.926–1.019, AD 0.954–1.103, controls 1.027–1.144) chan controls. Our data suggest that patients with DLB diagnosed by clinical criteria have changes in striatal post-synaptic D2 receptors. This may be of value in distinguishing DLB from AD during life.
Similar content being viewed by others
References
Byrne EJ, Lowe J, Lennox G, Godwin-Austen RB. Diffuse Lewy body disease; clinical features in 15 cases.J Neurol Neurosurg Psychiatry 1989; 52: 709–717.
Lennox G, Lowe J, Landon M, Byrne EJ, Mayer RJ, Godwin-Austen RB. Diffuse Lewy body disease: correlative neuropathology using anti-ubiquitin immunocytochemistry.J Neurol Neurosurg Psychiatry 1989; 52: 1236–1247.
Dickson DW, Crystal HA, Mattiace HA, et al. Diffuse Lewy body disease: light and electron microscopic immunocytochemistry of senile plaques.Acta Neuropathol (Berl) 1989; 78: 572–584.
McKeith IG. Senile dementia of Lewy body type — a review of clinical and pathological features: implications for treatment.Int J Geriatr Psychiatry 1995; 10: 919–926.
Hely MA, Reid WGJ, Halliday GM, et al. Diffuse Lewy body disease: clinical features in nine cases without coexistent Alzheimer's disease.J Neurol Neurosurg Psychiatry 1996; 60: 531–538.
Shergill S, Mullan L, D'Ath PJ, Katona CLE. What is the clinical prevalence of Lewy body dementia.Int J Geriatr Psychiatr 1994; 9: 907–912.
Byrne EJ, Lennox G, Godwin-Austen RB, et al. Diagnostic criteria for dementia associated with cortical Lewy bodies.Dementia 1991; 2: 283–284.
McKeith IG, Perry RH, Fairbairn AF, Jabeen S, Perry EK. Operational criteria for senile dementia of Lewy body type (SDLT).Pathol Med 1992; 22: 911–922.
Byrne EJ. Cortical Lewy body disease: an alternative view. In: Levy R, et al.Developments in dementia and functional disorders in the elderly. Petersfield, UK: Wrightson Biomedical, 1995:21–30.
McKeith IG, Fairbairn AF, Bothwell RA, et al. An evaluation of the predictive validity and inter-rater reliability of clinical diagnostic criteria for senile dementia of Lewy body type.Neurology 1994; 44: 872–877.
Förstl H, Sattel H, Bahro M. A1zheimer's disease: clinical features.Int Rev Psychiatry 1993; 5: 327–349.
McKeith IG, Galasko D, Wilcock GK, Byrne EJ. Lewy body dementia — diagnosis and treatment.Br J Psychiatry 1995; 167: 709–717.
McKeith IG. Cortical Lewy body disease: the view from Newcastle. In: Levy R, et al.Developments in dementia and functional disorders in the elderly. Petersfield, UK: Wrightson Biomedical; 1995: 9–20.
Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity.Brain 1991; 114: 2283–2301.
Koller WC. When does Parkinson's disease begin?Neurology 1992; 42: 27–31.
Perry EK, Marshall E, Perry RH, et al. Cholinergie and dopaminergic activities in senile dementia of Lewy body type.Alzheimer Dis Assoc Disord 1990; 4: 87–95.
Burn DJ, Mark MH, Playford ED, et al. Parkinson's disease in twins studied with18F-dopa and positron emission tomography.Neurology 1992; 42: 1894–1900.
Sawle GV, Wroe SJ, Lees AJ, Brooks DJ, Frackowiak RSJ. The identification of presymptomatic Parkinsonism: clinical and [18F]-dopa positron emission tomography studies in an Irish kindred.Ann Neurol 1992; 32: 609–617.
Tyrell PJ, Sawle GV, Ibanez V, et al. Clinical and positron emission tomographie studies in the “extrapyramidal syndrome” of dementia of Alzheimer's type.Arch Neurol 1990; 47: 1318–1328.
Brooks DJ. Functional imaging in relation to parkinsonian syndromes.J Neurol Neurosurg Psychiatry 1993; 115: 1–17.
Costa DC, Verhoeff NPLG, Cullum ID, et al. In vivo characterisation of 3-iodo-6-methoxybenzamide123I in humans.Eur J Nucl Med 1990; 16: 813–816.
McKhann G, Drachman D, Folstein MF, Katzman R, Price D, Standlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's disease.Neurology 1984: 34; 939–944.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinicopathological study of 100 cases.J Neurol Neurosurg Psychiatry 1992; 55: 181–184.
Folstein MF, Folstein SE, HcHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician.J Psychiatr Res 1975; 12: 189–198.
Pattie AH. A survey version of the clifton assessment procedures for the elderly (CAPE).Br Clin Psychol 1981; 20: 173–178.
Yesavage JA. Geriatric Depression Scale.Psychopharmacol Bull 1988; 24: 709–711.
Berl L. Clinical Dementia Rating (CDR).Psychopharmacol Bull 1988; 24: 637–639.
Roth M, Tym E, Mountjoy CQ, et al. CAMDEX: a standardised instrument for the diagnosis of mental disorders in the elderly with special reference to the early detection of dementia.Br J Psychiatry 1986; 149: 698–709.
Brücke T, Podreka I, Angelberger P, et al. Dopamine D2 receptor imaging with SPECT: studies in different neuropsychiatric disorders.J Cereb Blood Flow Metab 1991; 11: 220–228.
Brooks DJ, Ibanez J, Sawle GV, et al. Striatal D2 receptor status in patients with Parkinson's disease, striatonigral degeneration, and progressive supranuclear palsy, measured with11C-raclopride and positron emission tomography.Ann Neurol 1992; 31: 184–192.
Laulumaa V, Kuikka JT, Soininen H, Bergström K, Länsimies E, Riekkinen P. Imaging of D2 dopamine receptors of patients with Parkinson's disease using single photon emission tomography and iodobenzamide I 123.Arch Neurol 1993; 50: 509–512.
Pizzolato G, Chierichetti F, Rossato A, et al. Dopamine receptor SPET imaging in Parkinson's disease: a [123I]-IBZM and [99mTc]-HM-PAO study.Eur Neurol 1993; 33: 143–148.
Rinne UK, Laihinen A, Rinne JO, Någren K, Bergman J, Ruotsalainen U. Positron emission tomography demonstrates dopamine D2 receptor supersensitivity in the striatum of patients with early Parkinson's disease.Mov Disord 1990; 5: 55–59.
Rinne JO, Laihinen A, Rinne UK, Någren K, Bergman J, Ruotsalainen U. PET study on striatal dopamine D2 receptor changes during the progression of early Parkinson's disease.Mov Disord 1993; 8: 134–138.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Walker, Z., Costa, D.C., Janssen, A.G. et al. Dementia with Lewy bodies: a study of post-synaptic dopaminergic receptors with iodine-123 iodobenzamide single-photon mission tomography. Eur J Nucl Med 24, 609–614 (1997). https://doi.org/10.1007/BF00841397
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00841397