Abstract
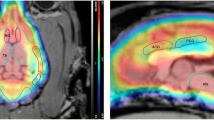
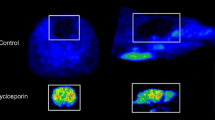
Radiolabelled 2β-Cabomethoxy-3β-(4-iodophenyl)tropane (β-CIT) has been used in clinical studies for the imaging of dopamine and serotonin transporters with single-photon emission tomography (SPET). 2β-Carbomethoxy-3β-(4-iodophenyl)nortropane (nor-β-CIT) is a des-methyl analogue of β-CIT, which in vitro has tenfold higher affinity (IC50=0.36 nM) to the serotonin transporter than β-CIT (IC50=4.2 nM). Nor-β-CIT may thus be a useful radioligand for imaging of the serotonin transporter. In the present study iodine-125 and carbon-11 labelled nor-β-CIT were prepared for in vitro autoradiographic studies on post-mortem human brain cryosections and for in vivo positron emission tomography (PET) studies in Cynomolgus monkeys. Whole hemisphere autoradiography with [125I]nor-β-CIT demonstrated high binding in the striatum, the thalamus and cortical regions of the human brain. Addition of a high concentration (1 μM) of citalopram inhibited binding in the thalamus and the neocortex, but not in the striatum. In PET studies with [11C]nor-β-CIT there was rapid uptake of radioactivity in the monkey brain (6% of injected dose at 15 min) and high accumulation of radioactivity in the striatum, thalamus and neocortex. Thalamus to cerebellum and cortex to cerebellum ratios were 2.5 and 1.8 at 60 min, respectively. The ratios obtained with [11C]nor-β-CIT were 20%–40% higher than those previously obtained with [11C]β-CIT. Radioactivity in the thalamus and the neocortex but not in the striatum was displaceable with citalopram (5 mg/kg). In conclusion, nor-β-CIT binds to the serotonin transporter in the primate brain in vitro and in vivo and has potential for PET and SPET imaging of the serotonin transporter in human brain.
Similar content being viewed by others
References
Rickels K, Schweizer E. Clinical overview of serotonin reuptake inhibitors.J Clin Psychiatry 1990; 51: 9–12.
Cash R, Raisman R, Ploska A, Agid Y. High and low affinity3H-imipramine binding sites in control and Parkinsonian brain.Eur J Pharmacol 1985; 117: 71–80.
Palmer AM, Francis PT, Benton JS, et al. Presynaptic serotonergic dysfunction in patients with Alzheimer's disease.J Neurochem 1987; 48: 8–15.
Battaglia G, Yeh SY, O'Hearn E, Molliver ME, Kuhar MJ, DeSouza EB. MDMA effects in the brain: pharmacological profile and evidence of neurotoxicity from neurochemical and autoradiographic studies.J Pharmacol Exp Ther 1987; 241: 911–916.
Ricaurte GA, Molliver ME, Martello MB, Katz JL, Wilson MA, Martello AL. Dexfenfluramine neurotoxicity in brains of non-human primates.Lancet 1990; 338: 1487–1488.
Neumeyer JL, Wang S, Milius RA, et al. [123I]2β-carbome-thoxy-3β-(4-iodophenyl)tropane: high-affinity SPECT radiotracer of monoamine reuptake sites in brain.J Med Chem 1991; 34: 3144–3146.
Kuikka JT, Bergström KA, Vanninen E, Laulumaa V, Hartikainen P, Länsimies E. Initial experience with SPET examinations using [123I]2β-carbomethoxy-3β-(4-iodophenyl)tropane ([123I]β-CIT) in human brain.Eur J Nucl Med 1993; 20: 783–786.
Brücke T, Kornhuber J, Angelberger P, Asenbaum S, Frassine H, Podreka I. SPECT imaging of dopamine and serotonin transporters with [123I]β-CIT. Binding kinetics in the human brain.J Neural Transm Gen Sect 1993; 94: 137–146.
Innis RB, Seibyl JB, Wallace E et al. SPECT imaging demonstrates loss of striatal dopamine transporters in Parkinson's disease.Proc Natl Acad Sci USA 1993; 90: 11965–11969.
Müller L, Halldin C, Farde L, et al. [11C]β-CIT, a cocaine analogue. Preparation, autoradiography and preliminary PET investigations.Nucl Med Biol 1993; 20: 249–255.
Farde L, Halldin C, Müller L, Suhari T Karlsson P, Hall H. PET study of [11C]β-CIT binding to monoamine transporters in the monkey and human brain.Synapse 1994; 16: 93–103.
Bergström KA, Kuikka JT, Ahonen A, Vanninen E. [123I]β-CIT, a tracer for dopamine reuptake sites, preparation and preliminary SPET studies in humans.J Nucl Biol Med 1994; 38 Suppl 1-4: 128–131.
Tiihonen J, Kuikka J, Bergström K, et al. Altered striatal dopamine re-uptake site densities in habitually violent and nonviolent alcoholics.Nature Med 1995; 1: 654–657.
Kuikka JT, Tiihonen J, Bergström KA, et al. Imaging of serotonin and dopamine transporters in the living human brain.Eur J Nucl Med 1995; 22: 346–350.
Bäekström I, Bergström M, Marcusson J. High affinity [3H]paroxetine binding to serotonin uptake sites in human brain tissue.Brain Res 1989; 486: 261–268.
Arranz B, Marcusson J. [3H]Paroxetine and [3H]citalopram as markers of the human brain 5-HT uptake site: a comparison study.J Neural Transm Gen Sect 1994; 97: 27–40.
Boja JW, Kuhar MJ, Kopajtic T, et al. Secondary amine analogues of 3β-(4′-substituted phenyl)tropane-2β-carboxylic acid esters andN-norcocaine exhibit enhanced affinity for serotonin and norepinephrine transporters.J Med Chem 1994; 37: 1220–1223.
Dorman GA, Kaczmarczyk SJ, Paxinos G, et al. Distribution of catecholamine uptake sites in human brain as determined by quantitative [3H]mazindol autoradiography.J Comp Neurol 1991; 304: 419–434.
Bäckström IT, Marcusson JO. High- and low-affinity [3H]desipramine-binding sites in human postmortem brain tissue.Neuropsychobiology 1990; 23: 68–73.
Swahn C-G, Halldin C, Günther I, Patt J, Ametamey S. Synthesis of unlabelled,3H- and125I-labelled β-CIT and its ω-fluoroalkyl analogues β-CITFE and β-CIT FP including the synthesis of precursors.J Label Cmpds Radiopharm 1996; 38: 675–685.
Lundkvist C, Halldin C, Swahn C-G, et al. Synthesis of11Cor18F-labelled analogues of β-CIT. Labelling in different positions and PET evaluation in Cynomolgus monkeys.J Label Cmpds Radiopharm 1995; 37: 52–54.
Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain.Neuropsychopharmacology 1994; 11: 245–256.
Hall H, Farde L, Halldin C, Hurd YL, Pauli S, Sedvall G. Autoradiographic localization of extrastriatal D2-dopamine receptors in the human brain using [125I]epidepride.Synapse 1996; 23: 115–123.
Persson A, d'Argy R, Gillberg P-G, et al. Autoradiography with saturation experiments of11C-Ro 15-1788 binding to human brain sections.J Neurosci Methods 1991; 36: 53–61.
Hyttel J. Citalopram: pharmacological profile of a specific serotonin uptake inhibitor with antidepressant activity.Prog Neuropsychopharmacol Biol Psychiatry 1982; 6: 277–295.
Andersen PH. The dopamine uptake inhibitor GBR12909: selectivity and molecular mechanisms of action.Eur J Pharmacol 1989; 166: 493–504.
Weinhard K, Dahlbom M, Eriksson L, et al. The ECAT EXACT HR: performance of a new high resolution positron scanner.J Comput Assist Tomogr 1994; 18: 110–118.
Karlsson P, Farde L, Halldin C, et al. PET examination of [11C]NNC 687 and [11C]NNC 756 as new radioligands for the D1-dopamine receptor.Psychopharmacology 1993; 113: 149–156.
Halldin C, Swahn C-G, Farde L, Sedvall G. Radioligand disposition and metabolism — key information in early drug development. In: Comar D, ed.PET for drug development and evaluation. Dordrecht: Kluwer; 1995: 55–65.
Bergström KA, Halldin C, Kuikka JT, et al. Lipophilic metabolite of [123I]β-CIT in human plasma may obstruct quantitation of the dopamine transporter.Synapse 1995; 19: 297–300.
Bergström KA, Halldin C, Lundkvist C, et al. Characterization of C-11 or I-123 labelled β-CIT-FP and β-CIT FE metabolism in monkey and human plasma. Identification of two labelled metabolites with HPLC.Hum Psychopharmacol 1996; 11: 483–490.
Swahn C-G, Halldin C, Farde L, Sedvall G. Metabolism of the PET ligand [11C]SCH 23390. Identification of two radiolabelled metabolites with HPLC.Hum Psychopharmacol 1994; 9: 25–31.
Marcusson J, Eriksson K. [3H]GBR-12935 binding to dopamine uptake sites in the human brain.Brain Res 1988; 457: 122–129.
Günther I, Hall H, Halldin C, Swahn C-G, Farde L, Sedvall G. Autoradiographic evaluation of [125I]β-CIT, [125I]β-CIT-FE and [125I]β-CIT FP binding in the human brain.Nucl Med Biol (in press)
Gatley SJ, Volkow ND, Fowler JS, Dewey SL, Logan J. Sensitivity of striatal [11C]cocaine binding to decreases in synaptic dopamine.Synapse 1995; 20: 137–144.
Dray A, Davies J, Oakley N, Tongroach P, Vellucci S. The dorsal and medial raphe projrections to the substantia nigra in the rat: electrophysical, biochemical and behavioral observations.Brain Res 1978: 151: 431–442.
Spampinato U, Esposito E, Samanin R. Serotonergic agonists reduce dopamine synthesis in the striatum only when the impulse flow of nigrostriatal neurons is intact.J Neurochem 1985; 980–982.
Ugedo L, Grenhoff G, Svensson TH. Ritanserin, a 5-HT2 receptor antagonist, activates midbrain dopamine neurons by blocking serotonergic inhibition.Psychopharmacology 1989; 98: 45–50.
Dewey SL, Smith GS, Logan J, et a1. Serotonergic modulation of striatal dopamine measured with positron emission tomography and in vivo microdialysis.J Neurosci 1995; 15: 821–829.
Suehiro M, Scheffel U, Dannals RF, Ravert HT. Ricaurte GA, Wagner HN Jr. A PET radiotracer for studying serotonin uptake sites: carbon-ll-McN-56522.J Nucl Med 1993; 34: 120–127.
Mazière B, Mazière M. Studying in vivo brain chemistry with SPECT: receptors and neurotransmission. In: Costa DC, Morgan GF, Lassen NA. eds.New trends in nculear neurology and psychiatry. London: John Libbey; 1993: 85–100.
Jagust WJ, Eberling JL, Roberts JA, et al. In vivo imaging of the 5-hydroxytryptamine reuptake site in primate brain using single photon emission computed tomography and [123I[5-iodo-6-nitroquipazin.Eur J Pharmacol 1993; 242: 189–193.
Szabo Z, Kao PF, Scheffel U. et al. Positron emission tomography imaging of serotonin transporters in the human brain using [11C](+)McN5652.Synapse 1995; 20: 37–43.
Jagust WJ, Eberlin JL, Biegon A, et al. Iodine-123-5-iodo-6-nitroquipazine: SPECT radiotracer to image the serotonin transporter.J Nucl Med 1996; 37: 1207–1214.
Misra AL, Pontani RB, Vadlamani NL. Metabolism of norcocaine,N-hydroxy norcocaine and cocaine-N-oxide in the rat.Xenobiotica 1979; 9: 189–199.
Jindal SP, Lutz T. Ion cluster techniques in drug metabolism: use of a mixture of labeled and unlabeled cocaine to facilitate metabolite identification.J Anal Toxicol 1986; 10: 150–155.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bergström, K.A., Halldin, C., Hall, H. et al. In vitro and in vivo characterisation of nor-β-CIT: a potential radioligand for visualisation of the serotonin transporter in the brain. Eur J Nucl Med 24, 596–601 (1997). https://doi.org/10.1007/BF00841395
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00841395