Summary
Previous studies have shown that osteoclasts obtained from chopped bones resorb surrogate calcified tissue substrata in vitro. These cultures contained all bone and marrow cell type pooled together. We have now parted the marrow from the bone and cultured the cells from the two fractions separately: on both resorbable substrates and on plastic in order to test their longevity in culture and ability to resorb following trypsinisation.
Marrow-fraction, bone-fraction or whole bone derived cells were harvested from prehatch chick long bone shafts after removing the periosteum; seeded on sperm whale dentine (SWD) slices or plastic dishes and cultured continously, or trypsinised and reseeded on to fresh substrata at weekly or half-weekly intervals. Observations were made by light microscopy and SEM.
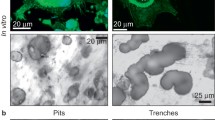
Many multinucleate cells were observed in the marrow fraction immediately after settling, deriving presumably from poorly adherent osteoclasts, next to bone, which had not been resorbing at the time of harvesting. By three days in culture on plastic, multinucleate cells were very large both in terms of plant extent and nuclear number: cell fusion occurred between osteoclasts and between osteoclasts and small, round uninuclear cells. SWD was extensively resorbed.
The adherence of the osteoclasts was greater (a) to plasuc upon trypsinisation than that of the other cells; and (b) to SWD than to plastic, particularly if the cells were resorbing. Trypsinised cells regained their resorptive capacity after seeding on to new SWD, but only for 1 or 2 treatments.
Bone derived cells were similar to the marrow cultures, except for the much higher proportion of other bone cell types. Trypsinisation caused a higher proportional loss of multinucleate cells from both SWD and plastic.
Resorption was still occurring at 6 weeks in all cultures. A wide diversity existed in the shapes, depths, plan areas and volumes of the resorption pits.
Similar content being viewed by others
References
Boyde A (1984) Methodology of calcified tissue specimen preparation for SEM. In: Dickson GR (ed) Methods of calcified tissue preparation. Elsevier, Amsterdam, pp 251–307
Boyde A, Jones SJ (1979) Estimation of the size of resorption lacunae in mammalian calcified tissues using SEM stereophotogrammetry. Scanning Electron Microscopy/1979/II, SEM Inc-AMF O'Hare, pp 393–402
Boyde A, Jones SJ (1983) Backscattered electron imaging of skeletal tissue. Metab Bone Dis Relat Res 5:145–150
Boyde A, Jones SJ (1985) Bone modelling in the implantation bed. J Biomed Mater Res 19:199–224
Boyde A, Ali NN, Jones SJ (1983) Computer aided measurement of resorbtive activity of isolated osteoclasts. Proc Roy Microsc Soc 18:357 (abstr)
Boyde A, Ali NN, Jones SJ (1984) Resorption of dentine by isolated osteoclasts in vitro. Brit Dent J 156:216–220
Boyde A, Ali NN, Jones SJ (1985) Optical and scanning electron microscopy in the single osteoclast resorption assay. Scanning Electron microscopy/1985/III, SEM Inc-AMF O'Hare, pp 1259–1271
Burger EH, Van der Meer JWM, Van de Gevel JS, Gribnau JC, Thesingh CW, Van Furth R (1982) In vitro formation of osteoclasts from long term cultures of bone marrow mononuclear phagocytes. J Exp Med 156:1604–1614
Chambers TJ (1985) The pathobiology of the osteoclast. J Clin Pathol 38:241–252
Cambers TJ (1985) The pathobiology of the osteoclast. J Clin Pathol 38:241–252
Chambers TJ, Magnus CJ(1982) Calcitonin alters behaviour of isolated osteoclasts. J Pathol 136:27–39
Chambers TJ, Revel PA, Fuller K, Athanasou NA (1984) Resorption of bone by isolated rabbit osteoclasts. J Cell Sci 66:383–399
Horton MA, Rimmer EF, Chambers TJ (1985) Surface marker and functional characterisation of multinucleate giant cells generated in long term culture of neonatal rabbit bone marrow. Bone [in press — Abstract — Bone and Tooth Soc, London Oct 1985]
Jaworski ZFG, Duck B, Sekaly G (1981) Kinetics of osteoclasts and their nuclei in evolving secondary Haversian systems. J Anast 133:397–405
Jones SJ, Boyde A (1977) Some morphological observations on osteoclasts. Cell Tissue Res 185:387–397
Jones SJ, Boyde A, Ali NN (1984) The resorption of biological and non-biological substrates by cultured avian and mammalian osteoclasts. Anat Embryol 170:247–256
Jones SJ, Boyde A, Ali NN, Maconnachie E (1985a) A review of bone cell and substratum interactions. Scanning 7:5–24
Jones SJ, Ali NN, Maconnachie E, Boyde A (1985b) Normal variation in osteoclastic activity. Calcif Tissue Int [Suppl] 38:S5
Jones SJ, Maroudas NM, Ali NN, Boyde A (1985c) The development of chemically defined substrata for testing osteoclast function. Calcif Tissue Int [Suppl] 38:S5
Loutit JF, Townsend KMS (1982b) Longevity of osteoclasts in radiation chimaeras of beige and osteopetrotic microphthalmic mice. Brit J Exp Pathol63:214–220
Loutit JF, Townsend KMS (1982b) Longevity of osteoclasts in radiation chimaeras of osteopetrotic beige and normal mice. Brit J Exp Pathol 63:221–223
Marks SC (1984) Congenital osteopetrotic mutations as probes of the origin, structure, and function of osteoclasts. Clin Orthop 189:239–263
Marks SC, Schneider GB (1982) Transformations of osteoclast phenotype in a rats cured of congenital osteopetrosis. J Morphol 174:141–147
Marks SC, Walker DG (1981) The hematogenous origin of osteoclasts: Experimental evidence from osteopetrotic (microphthalmic) mice treated with spleen cells from beige mouse donors. Am J Anat 161:1–10
Osdoby P, Martini MC, Caplan AI (1982) Isolated osteoclasts and their presumed progenitor cells, the monocyte, in culture. J Exp Zool 224:331–344
Schneider GB (1985) Cellular specificity of the cure for osteopetrosis: isolation and treatment with pluripotent hemopoietic stem cells. Bone 6:241–247
Spooncer E, Dexter TM (1984) Long term bone marrow cultures. Bibl Haematologica, no. 48. Karger, Basel, pp 366–383
Suda T, Testa NG, Allen TD, Onions D, Jarrett O (1983) Effect of hydrocortisone on osteoclasts generated in cat bone marrow cultures. Calcif Tissue Int 35:82–86
Zambonin-Zallone A, Teti A, Primavera MV (1982) Isolated osteoclasts in primary culture: first observations on structure and survival in culture media. Anat Embryol 165:405–413
Zambonin-Zallone A, Teti A, Primavera MV (1984) Resorption of vital or devitalized bone by isolated osteoclasts in vitro. Cell Tissue Res 235:561–564
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jones, S.J., Ali, N.N. & Boyde, A. Survival and resorptive activity of chick osteoclasts in culture. Anat Embryol 174, 265–275 (1986). https://doi.org/10.1007/BF00824342
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00824342