Summary
Deep-etching and rotary-shadowing techniques were used to describe crossbridges in fish (Chanda ranga) muscle, relaxed and in iodoacetate rigor conditions. Three major fracture planes from rigor muscle were studied using stereomicroscopy and Fourier image analysis.
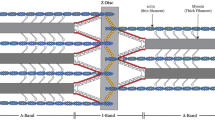
The 1,0 plane reveals alternating thick and thin filaments with the thick filaments frontmost in the fracture and the thin filaments in the recessed plane. All crossbridges coming from the frontmost thick filaments are visible on actin filaments in the 1,0 plane. Fourier transforms of digitized images from these fracture planes exhibit axial periodicities of 14 and 36 nm. The actin layer, a fracture plane just below the myosin filaments in the 1,0 plane, shows end-on views of crossbridges projecting out of the fracture plane and limited transverse alignment of crossbridges. Actin layer Fourier transforms demonstrate a 14 nm reflection associated with the attachment of crossbridges with a mean axial periodicity determined by their myosin origins. The 1,1 lattice direction shows pairs of thin filaments alternated with single thick filaments. In this view, all crossbridges coming from three adjacent myosins are visible.
In all fracture planes, decoration of individual thin filaments by crossbridges is variable, but usually one (singlet) or two (doublet) closely spaced crossbridges mark each actin target zone, at intervals of 35–38 nm. Counts of crossbridges decorating actin filaments give an average of four every three target zones. The anticipated stagger of target zones for crossbridges from two adjacent myosin filaments is observed. Alignment of actin target zones across the sarcomere is good. We can distinguish two distinct shapes for rigor crossbridges: a narrow, straight bridge and a wider bridge with a triangular shape. We interpret these as being the appearance of crossbridges with one or two S1 subfragments (single and double headed) respectively. Comparison between rigor and relaxed structures indicates attachment of all crossbridges in rigor.
Similar content being viewed by others
References
CASTELLANI, L., VIBERT, P. & COHEN, C. (1983) Structure of myosin/paramyosin filaments from a molluscan muscle.J. molec. Biol. 167, 853–72.
CHALOVICH, J. M., CHOCK, P. R. & EISENBERG, E. (1981) Mechanism of action of troponin-tropomyosin: Inhibition of actomyosin ATPase activity without inhibition of myosin binding to actin.J. biol. Chem. 256, 575–8.
COOKE, R. & FRANKS, K. (1980) All myosin heads form bonds with actin in rigor rabbit skeletal muscle.Biochemistry 19, 2265–9.
CRAIG, R., SZENT-GYÖRGYI, A. G., BEESE, L., FLICKER, P., VIBERT, P. & COHEN, C. (1980) Electron microscopy of thin filaments decorated with a Ca2+-regulated myosin.J. molec. Biol. 140, 35–55.
EISENBERG, E. & GREENE, L. E. (1980) The relation of muscle biochemistry to muscle physiology.Ann. Rev. Physiol. 42, 293–309.
FRANZINI-ARMSTRONG, C., VARRIANO-MARSTON, E. & HASELGROVE, J. C. (1983) Crossbridges in vertebrate muscle.Biophys. J. 41, 98a.
FREUNDLICH, A., LUTHER, P. K. & SQUIRE, J. M. (1980) High-voltage electron microscopy of crossbridge interactions in striated muscle.J. Musc. Res. Cell Motility 1, 321–43.
FREY, T. G., COSTELLO, M. J., KARLSSON, B., HASELGROVE, J. C. & LEIGH, J. S. (1982) Structure of the cytochrome C oxidase dimer. Electron microscopy of two dimensional crystals.J. molec. Biol. 162, 113–30.
GILLIS, J. M. & O'BRIEN, E. J. (1975) The effect of calcium ions on the structure of reconstituted muscle thin filaments.J. molec. Biol. 99, 445–59.
GREENE, L. E. & EISENBERG, E. (1980a) Dissociation of the actin-subfragment 1 complex by adenylyl-5′ imidodiphosphate, ADP and Pi.J. biol. Chem. 255, 543–8.
GREENE, L. E. & EISENBERG, E. (1980b) The binding of heavy meromyosin to F-actin.J. biol. Chem. 255, 549–54.
HARRINGTON, W. F., SUTOH, K. & CHIAO, Y.-C. C. (1979) Myosin filaments and cross-bridge movement. InMotility in Cell Function (edited by PEPE, F. A., SANGER, J. W. and NACHMIAS, V. T.), pp. 69–90. New York: Academic Press.
HASELGROVE, J. C. (1975) X-ray evidence for conformational changes in the myosin filaments of vertebrate striated muscle.J. molec. Biol. 92, 113–43.
HASELGROVE, J. C. (1980) A model of myosin crossbridge structure consistent with the low-angle X-ray diffraction patterns of vertebrate muscle.J. Musc. Res. Cell Motility 1, 177–91.
HASELGROVE, J. C. (1983) Structure of vertebrate striated muscle as determined by X-ray diffraction studies. InHandbook of Physiology, Section 10,Skeletal Muscle (edited by PEACHEY, L. D. and ADRIAN, R. H.), pp. 143–72. Baltimore: Williams and Wilkins.
HASELGROVE, J. C. & REEDY, M. K. (1978) Modeling rigor cross-bridge patterns in muscle. I. Initial studies of the rigor lattice of insect flight muscle.Biophys. J. 24, 713–28.
HASELGROVE, J. C. & REEDY, M. K. (1984) Geometrical constraints affecting crossbridge formation in insect flight muscle.J. Musc. Res. Cell Motility 5, 3–24.
HEUSER, J. E. (1983) Structure of the myosin cross bridge lattice in insect flight muscle.J. molec. Biol. 169, 123–54.
HEUSER, J. E. & COOKE, R. (1983) Actin-myosin interaction visualized by the quick-freeze, deep-etch replica technique.J. molec. Biol. 169, 97–122.
HEUSER, J. E. & SALPETER, S. R. (1979) Organization of acetylcholine receptors in quick-frozen deep-etched and rotary replicated torpedo postsynaptic membrane.J. Cell Biol. 82, 150–73.
HOLMES, K. C., TREGEAR, R. T. & BARRINGTON-LEIGH, J. (1980) Interpretation of the low angle X-ray diffraction from insect flight muscle in rigor.Proc. R. Soc. Lond. Ser. B 207, 13–33.
HUXLEY, H. E. (1957) The double array of filaments in cross-striated muscle.J. Biophys. Biochem. Cytol. 3, 631–47.
HUXLEY, H. E. & BROWN, W. (1967) The low angle X-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor.J. molec. Biol. 30, 383–434.
HUXLEY, H. E., FARUQI, A. R., KRESS, M., BORDAS, J. & KOCH, M. H. J. (1982) Time-resolved X-ray diffraction studies of the myosin layer-line reflections during muscle contraction.J. molec. Biol. 158, 637–84.
IP, W. & HEUSER, J. E. (1983) Direct visualization of the myosin cross bridge helices on relaxed rabbit psoas thick filaments.J. molec. Biol. 171, 105–9.
KENSLER, R. W. & STEWART, M. (1983) Frog skeletal muscle thick filaments are three stranded.J. Cell Biol. 96, 1797–802.
KEYNES, R. D. & MARTINS-FERREIRA, H. (1953) Membrane potentials in the electroplates of the electric eel.J. Physiol. 119, 315–51.
LOCKWOOD, A. P. M. (1961) ‘Ringer’ solutions and some notes on the physiological basis of their ionic composition.Comp. Biochem. Physiol. 2, 241–89.
MAGID, A. & REEDY, M. K. (1980) X-ray diffraction observations of chemically skinned frog skeletal muscle processed by an improved method.Biophys. J. 30, 27–40.
MAW, M. C. & ROWE, A. J. (1980) Fraying of A-filaments into three subfilaments.Nature 286, 412–4.
MILLER, A. & TREGEAR, R. T. (1972) Structure of insect fibrillar flight muscle in the presence and absence of ATP.J. molec. Biol. 70, 85–104.
OFFER, G., COUCH, J., O'BRIEN, E. & ELLIOTT, A. (1981) Arrangement of crossbridges in insect flight muscles in rigor.J. molec. Biol. 151, 663–702.
OFFER, G. & ELLIOT, A. (1978) Can a myosin molecule bind to two actin filaments?Nature 271, 325–9.
PAGE, S. G. & HUXLEY, H. E. (1963) Filament lengths in striated muscle.J. Cell Biol. 19, 369–90.
PEPE, F. A. (1982) The structure of vertebrate skeletal muscle myosin filaments. InCell and Muscle Motility, Vol. 2 (edited by DOWBEN, R. M. and SHAY, J. W.), pp. 141–71. New York: Plenum Press.
REEDY, M. K. (1967) Cross-bridges and periods in insect flight muscle.Am. Zool. 7, 465–81.
REEDY, M. K. (1968) Ultrastructure of insect flight muscle. I. Screw sense and structural grouping in rigor cross-bridge lattice.J. molec. Biol. 31, 155–76.
REEDY, M. K. & BARKAS, A. E. (1974) Disordering of myofibril structure due to fixation, dehydration and embedding.J. Cell Biol. 63, 282a.
REEDY, M. K. & GARRETT, W. E. (1977) Electron and microscopic studies ofLethocerus flight muscle in rigor. InInsect Flight Muscle (edited by TREGEAR, R. T.), pp. 115–35. Amsterdam: Elsevier-North Holland.
REEDY, M. K., GOODY, R. S., HOFMAN, W. & ROSENBAUM, G. (1983) Co-ordinated electron microscopy and X-ray studies of glycerinated insect flight muscle. I. X-ray diffraction monitoring during preparation for electron microscopy of muscle fibres fixed in rigor, in ATP and in AMPPNP.J. Musc. Res. Cell Motility 4, 25–53.
REEDY, M. K., HOLMES, K. C. & TREGEAR, R. T. (1965) Induced changes in the orientation of the cross-bridges of glycerinated insect flight muscle.Nature 207, 1276–80.
SJOSTROM, M. & SQUIRE, J. M. (1977) Cryo-ultramicrotomy and myofibrillar fine structure: a review.J. Microsc. 111, 239–78.
SQUIRE, J. M. (1972) General model of myosin filament structure.J. molec. Biol. 72, 125–38.
SQUIRE, J. M. (1973) General model of myosin filament structure: III. Molecular packing arrangements in myosin filaments.J. molec. Biol. 77, 291–323.
SQUIRE, J. M. (1979) InFibrous Proteins Scientific, Industrial and Medical Aspects (edited by FARRY, D. A. D. and CREAMER, L. K.), Vol. I, pp. 27–70. London: Academic Press.
SQUIRE, J. M. (1981)The Structural Basis of Muscle Contraction. New York: Plenum Press.
STEWART, M., KENSLER, R. W. & LEVINE, R. J. C. (1981) Structure ofLimulus telson muscle thick filaments.J. molec. Biol. 153, 781–90.
TAYLOR, K. A. & AMOS, L. A. (1981) A new model for the geometry of the binding of myosin cross-bridges to muscle thin filaments.J. molec. Biol. 147, 297–324.
THOMAS, D. D. & COOKE, R. (1980) Orientation of spin-labeled myosin heads in glycerinated muscle fibers.Biophys. J. 32, 891–906.
THOMAS, D. D., ISCHIWATA, S., SEIDEL, J. C. & GERGELY, J. (1980) Submillisecond rational dynamics of spin-labelled myosin heads in myofibrils.Biophys. J. 32, 873–90.
TRINICK, J. A. (1981) End-filaments: A structural element of vertebrate skeletal muscle thick filaments.J. molec. Biol. 151, 309–14.
TRINICK, J. A. & OFFER, G. (1979) Cross-linking of actin filaments by heavy meromyosin.J. molec. Biol. 133, 549–56.
VIBERT, P. & CRAIG, R. (1982) Three-dimensional reconstruction of thin filaments decorated with a Ca2+-regulated myosin.J. molec. Biol. 157, 299–319.
VIBERT, P. & CRAIG, R. (1983) Electron microscopy and image analysis of myosin filaments from scallop striated muscle.J. molec. Biol. 165, 303–20.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Varriano-Marston, E., Franzini-Armstrong, C. & Haselgrove, J.C. The structure and disposition of crossbridges in deep-etched fish muscle. J Muscle Res Cell Motil 5, 363–386 (1984). https://doi.org/10.1007/BF00818256
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00818256