Abstract
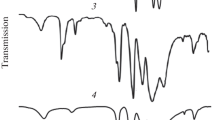
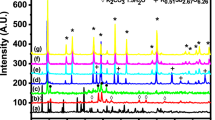
Potassium/ammonium salts of 12-molybdophosphoric acid were modified by the addition of an Sb5+ salt. The addition of antimony led to a remarkable increase in the thermal structural stability of the compounds obtained. The incipient destruction of the Keggin unit in the mixed potassium/ammonium salt of 12-molybdophosphoric acid was shifted from 400–420°C to 500°C. The potassium salt modified by the addition of one Sb5+ atom per KU decomposed at temperatures higher than 600°C. This property allowed the samples to be used as catalysts for high temperature, gas-phase oxidation reactions, such as the oxidehydrogenation of ethane. The compounds did not undergo structural decomposition at temperatures as high as 540°C under reaction conditions, but were poorly active and selective in ethylene formation. Therefore, antimony-stabilized compounds were further modified by the addition of transition metal ions in order to improve the catalytic performance. The addition of small amounts of iron, chromium and cerium ions led to an improvement of the catalytic performance; the compound was apparently monophasic, and characterized by the cubic crystalline structure typical of the salts of 12-molybdophosphoric acid.
Similar content being viewed by others
References
N.Z. Tkhiep, P.A. Shiryaev, M.Yu. Kutyrev, O.V. Krylov and I.N. Staroverova, Kinet. Catal. 32 (1991) 634.
S. Albonetti, F. Cavani, M. Koutyrev and F. Trifirò, in:Heterogeneous Catalysis and Fine Chemicals III, eds. M. Guisnet et al. (Elsevier, Amsterdam, 1993) p. 471.
V. Ernst, Y. Barbaux and P. Courtine, Catal. Today 1 (1987) 167.
O. Watzenberger, G. Emig and D.T. Lynch, J. Catal. 124 (1990) 247.
G. Centi, V. Lena, F. Trifirò, D. Ghoussoub, C.F. Aissi, M. Guelton and J.P. Bonnelle, J. Chem. Soc. Faraday Trans. 86 (1990) 2775.
G. Centi and F. Trifirò, in:Catalytic Science and Technology, Vol. 1 (Kodansha, Tokyo, 1991) p. 225.
M.A. Fedotov, L.G. Detusheva, L.I. Kuznetsova and L.A. Likholobov, Russ. J. Inorg. Chem. 38 (1993) 515.
M. Faraj and C.L. Hill, J. Chem. Soc. Chem. Comm. (1987) 1487.
M.W. Droege and R.G. Finke, J. Mol. Catal. 69 (1991) 323.
M. Akimoto, Y. Tsuchida, K. Sato and E. Echigoya, J. Catal. 72 (1981) 83.
J. Fisher, L. Ricard and R. Weiss, J. Am. Chem. Soc. 98 (1976) 3050.
M. Michelon, G. Hervé and M. Leyrie, J. Inorg. Nucl. Chem. 42 (1980) 1583.
P. Souchay, M. Leray and G. Hervé, Compt. Rend. Acad. Sci. Ser. C 271C (1970) 1337.
G.C. Bhattacharya and S.K. Roy, J. Indian Chem. Soc. 50 (1973) 359.
M.A. Fedotov, L.G. Detusheva, L.I. Kuznetsova and V.A. Likholobov, Russ. J. Inorg. Chem. 38 (1993) 477 (Engl. transl.).
S. Albonetti, F. Cavani, F. Trifirò, M. Gazzano, M. Koutyrev, F.C. Aissi, A. Aboukais and M. Guelton, J. Catal. 146 (1994) 491.
S. Albonetti, F. Cavani, E. Etienne, M. Favaro, A. Galli, F. Trifirò and M. Koutyrev, in preparation.
R. Fricke and G. Ohlmann, J. Chem. Soc. Faraday Trans. I 82 (1986) 273.
F. Cavani, F. Trifirò and M. Koutyrev, Ital. Patent 003150/91, assigned to Eniricerche.
Author information
Authors and Affiliations
Additional information
On leave from the Institute of Chemical Physics, Academy of Sciences, Russia.
Rights and permissions
About this article
Cite this article
Albonetti, S., Cavani, F., Trifirò, F. et al. On the antimony-stabilized cubic structure of potassium/ammonium salts of 12-molybdophosphoric acid and its catalytic performance in the oxidehydrogenation of ethane. Catal Lett 30, 253–262 (1994). https://doi.org/10.1007/BF00813691
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00813691