Abstract
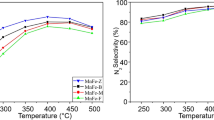
The N2O decomposition over an [Fe]-ZSM-5 and an Fe-HZSM-5 zeolite was studied. We found that framework incorporated iron species were much more active than Fe(III) introduced as framework charge countercations by ion exchange (TOF at 0.1 vol% N2O:1.47 × 10−4 at 280°C for [Fe]-ZSM-5 vs. 2.58 × 10−4 at 468°C for Fe-HZSM-5). The higher activity of [Fe]-ZSM-5 was attributed to the uniqueness of framework iron species. Both [Fe]-ZSM-5 and Fe-HZSM-5 zeolites showed enhanced activity in the presence of excess oxygen. This is in sharp contrast to ruthenium exchanged zeolites which showed strong oxygen inhibiting effect on the rate of N2O decomposition.
Similar content being viewed by others
References
C.M. Fu, V.N. Korchak and W.K. Hall, J. Catal. 68 (1981) 166.
J. Leglise, J.O. Petunchi and W.K. Hall, J. Catal. 86 (1984) 392.
L.M. Aparicio, M.A. Ulla, W.S. Milman and J.A. Dumesic, J. Catal. 110 (1988) 330.
G.I. Panov, V.I. Sobolev and S. Kharitonov, J. Mol. Catal. 61 (1990) 85.
M. Tabata, H. Hamada, Y. Kindachi, M. Sasaki and T. Ito, Chem. Exp. 7 (1992) 77.
Y.-J. Li and J.N. Armor, Appl. Catal. B 1 (1992) L21–29.
Y.-J. Li and J.N. Armor, US Patent 5171553 (1992).
Y.-F. Chang, J.G. McCarty, E.D. Wachsman and V. Wong, Appl. Catal. B 84 (1994) 283.
V.I. Sobolev, O.N. Kovalenko, A.S. Kharitonov, Y.D. Pankratiev and G.I. Panov, Mendeev's Commun. 1 (1991) 29.
V.I. Sobolev, G.I. Panov, A.S. Kharitonov, V.N. Romannikov, A.M. Volodin and K.G. Ione, J. Catal. 139 (1993) 435.
R. Szostak and T.L. Thomas, J. Catal. 100 (1986) 555.
G.P. Handreck and T.D. Smith, J. Chem. Soc. Faraday Trans. I 85 (1989) 3195.
Y.-L. Zhang, PhD Thesis, Fudan University, PR China (1994).
A. Meagher, V. Nair and R. Szostak, Zeolites 8 (1988) 3.
M.A. Uddin, T. Komatsu and T. Yoshima, Microporous Mater. 1 (1993) 201.
J. Galuszka, T. Sato and J.K. Sawacki, J. Catal. 136 (1992) 96.
M.A. Uddin, T. Komatsu and T. Yoshima, J. Catal. 146 (1994) 468.
T. Inui, H. Matsuda, O. Yamase, H. Nagata, K. Fukuda, T. Ukawa and A. Miyamota, J. Catal. 98 (1986) 491.
P.A. Jacobs,Carboniogenic Activity of Zeolites (Elsevier, Amsterdam, 1977).
O.D. Delafosse, in:Catalysis by Zeolites, eds. B. Imelik et al. (Elsevier, Amsterdam, 1980) p. 235.
P.A. Jacobs, J.B. Utterhoeven and H.K. Beyer, J. Chem. Soc. Faraday Trans. I 75 (1979) 56.
R.G. Herman, J.H. Lunsford, H.K. Beyer, P.A. Jacobs and J.B. Utterhoeven, J. Phys. Chem. 79 (1975) 2388.
P.A. Jacobs, H. Nijs and J. Verdonck, J. Chem. Soc. Faraday Trans. I 75 (1979) 1196.
Y.-Y. Huang and J.R. Anderson, J. Catal. 40 (1975) 143.
R.L. Garten, W.N. Delgass and M. Boudart, J. Catal. 18 (1970) 90.
W.N. Delgass, R.L. Garten and M. Boudart, J. Phys. Chem. 73 (1969) 2970.
K.G. Ione, L.A. Vostrikova and M.W. Mastikin, J. Mol. Catal. 31 (1985) 355.
L.M. Kustov, V.B. Kazansky and P. Ratnasamy, Zeolites 7 (1987) 79.
S. Kaliaguine, J.B. Nagy and Z. Gabelica, in:Keynotes in Energy Related Catalysis, ed. S. Kaliaguine (Elsevier, Amsterdam, 1988) p. 381.
A. Raj, S. Sivasanker and K. Lázár, J. Catal. 147 (1994) 207.
R. Szostak, N. Nair, D.K. Simmons, T.L. Thomas, R. Kuvadia, B. Dunson and D.C. Shieh, in:Innovations in Zeolite Materials Science, eds. P.J. Grobert et al. (Elsevier, Amsterdam, 1988) p. 403.
P.A. Jacobs, in:Metal Clusters in Catalysis, eds. B.C. Gates, L. Guczi and H. Knözinger (Elsevier, Amsterdam, 1986) p. 357.
E.R.S. Winter, J. Catal. 15 (1969) 144.
E.R.S. Winter, J. Catal. 19 (1970) 32.
G.M. Dhar and V. Srinivasan, Int. J. Chem. Kinet. 14 (1982) 415.
Y.-F. Chang, J.G. McCarty and E.D. Wachsman, Appl. Catal. B, submitted.
G.I. Panov, A.S. Kharitonov and V.I. Sobolev, Appl. Catal. A 98 (1993) 1.
Y.-F. Chang, G.A. Somorjai and H. Heinemann, J. Catal., accepted (1994).
B. Wichterlova, Zeolites 1 (1981) 181.
A.V. Kucherov and A.A. Slinkin, Zeolites 6 (1986) 1754.
A.V. Kucherov, A.A. Slinkin, G.K. Beyer and G. Berbely, J. Chem. Soc. Faraday Trans. I 85 (1989) 1989.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chang, Y.F., McCarty, J.G. & Zhang, Y.L. N2O decomposition over [Fe]-ZSM-5 and Fe-HZSM-5 zeolites. Catal Lett 34, 163–177 (1995). https://doi.org/10.1007/BF00808332
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00808332