Summary
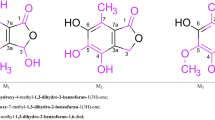
X-ray structural data are reported for 2-hydroxythiobenzoic acid (T=200 K;P21/a;a=14.903(5) Å,b=5.203(3) Å,c=9.114(6) Å, β=92.40(4)°;Z=4;R=0.049) and 2-hydroxydithiobenzoic acid (T=297 K;P21/a;a=14.416(3) Å,b=13.447(3) Å,c=3.947(1) Å, β=90.96(2)°;Z=4;R=0.047). In 2-hydroxythiobenzoic acid, each two molecules form cyclic dimersvia S-H...O=C hydrogen bonds, analogous to the association pattern of 2-hydroxybenzoic acid. In 2-hydroxydithiobenzoic acid, the molecules are linked to chains by S-H...O(H)-C hydrogen bonds. Solid state IR, and solution IR and NMR spectroscopic data of 2-hydroxybenzoic acid, 2-hydroxythiobenzoic acid, and 2-hydroxydithiobenzoic acid are summarized. The main characteristics of the intramolecularly associated phenolic O-H groups of the three title compounds are\(\bar v_{OH} = 3230, 3120, 2750 cm^{--1} \) for the solids,\(\bar v_{OH} = 3210, 3185, 2945 cm^{--1} \) for solutions (CCl4), and δOH=10.21, 10.53, 12.20 ppm for solutions (CCl4:CDCl3=5:1).
Zusammenfassung
Röntgenstrukturdaten von 2-Hydroxythiobenzoesäure (T=200 K;P21/a;a=14.903(5) Å,b=5.203(3) Å,c=9.114(6) Å, β=92.40(4)°;Z=4;R=0.049) und 2-Hydroxydithiobenzoesäure (T=297 K;P21/a;a=14.416(3) Å,b=13.447(3) Å,c=3.947(1) Å, β=90.96(2)°;Z=4;R=0.047) werden berichtet. In 2-Hydroxythiobenzoesäure sind jeweils zwei Moleküle über S-H...O=C Wasserstoffbrückenbindungen zu zyklischen Dimeren verbunden, in Analogie zum Assoziationsmuster von 2-Hydroxybenzoesäure. In 2-Hydroxydithiobenzoesäure sind die Moleküle hingegen über S-H...O(H)-C Wasserstoffbrückenbindungen zu Ketten verknüpft. Festkörper-IR-, und Lösungs-IR-und NMR-spektroskopische Daten von 2-Hydroxybenzoesäure, 2-Hydroxythiobenzoesäure und 2-Hydroxydithiobenzoesäure werden gegenübergestellt. Die wesentlichen Charakteristika der intramolekular assoziierten phenolischen O-H Gruppen der drei Titelverbindungen sind\(\bar v_{OH} = 3230, 3120, 2750 cm^{--1} \) für die Festkörper sowie\(\bar v_{OH} = 3210, 3185, 2945 cm^{--1} \) und δOH=10.21, 10.53, 12.20 ppm für Lösungen (CCl4 bzw. CCl4:CDCl3=5:1).
Similar content being viewed by others
References
Bacon G. E., Jude R. E. (1973) Z. Krist.138: 19
Wojcik M. J., Pawluszkiewicz C. (1983) Can. J. Chem.61: 1449
Derkosch J., Mikenda W., Steinwender E. (1987) Spectrochim. Acta43A: 823
Chem. Fabr. van Heyden (1923) Friedländer14: 1236
Sheldrick G. M. (1976) SHELX76, Program for crystal structure determination, Univ. of Cambridge, England
Krebs B. (1983) Angew. Chem.95: 113
Miyamae H, Oikawa T. (1985) Acta Cryst. C41: 1489
Behr A., Keim W., Kipshagen W., Vogt D., Herdtweck D., Herrmann W. A. (1988) J. Organomet. Chem.344: C15
Steinwender E., Mikenda W. (1990) Monatsh. Chem.121: 809
Mikenda W., Pertlik F., Steinwender E. (1993) Monatsh. Chem.124: 867
Pertlik F., Mikenda W., Steinwender E. (1993) J. Crystallogr. Spectrosc. Res.23: 389
Mereiter K., Mikenda W., Steinwender E. (1993) J. Crystallogr. Spectrosc. Res.23: 397
Steinwender E., Mikenda W. (1994) Monatsh. Chem.125: 695
Author information
Authors and Affiliations
Additional information
Dedicated to Prof.O. Olaj on the occasion of his 60th birthday
Rights and permissions
About this article
Cite this article
Mikenda, W., Steinwender, E. & Mereiter, K. Hydrogen bonding in 2-hydroxybenzoic, 2-hydroxythiobenzoic, and 2-hydroxydithiobenzoic acid. A structural and spectroscopic study. Monatsh Chem 126, 495–504 (1995). https://doi.org/10.1007/BF00807422
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00807422