Abstract
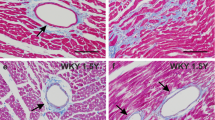
Hyperplasia of myocytes in cardiac adaptation is a rare event in the mammalian cardiac muscle. Recent findings support the concept that proliferation of myocytes in the adult mammalian heart may be induced after a prolonged increase in pressure load on the myocardium. To determine whether short-term hypertension leads to hyperplasia of myocyte nuclei in the rat heart renal hypertension was produced in 12 Wistar rats. As soon as hypertension occurred, bromodeoxyuridine (BrdU) (50 mg/kg/day) was injected intraperitoneally on three subsequent days. Twelve sham-operated rats served as controls. After 3 days, the left cardiac ventricle was excised and double-staining with anti-BrdU antibody and propidium iodide was performed to determine the phase of cell-cycle of the BrdU-positive cells by flow-cytometry. Immunohistochemical double-staining with desmin, smooth muscle actin, vimentin, and BrdU was done to classify the BrdU-positive cells.
Most of the BrdU-positive cells were in the G0/G1-phase of the cellcycle, suggesting cell proliferation or DNA-repair have taken place; polyploidy was not observed. In the hypertensive group (4.62%±2.36) significantly more cells incorporated BrdU than in the control group (1.46%±0.96). Immunohistochemically, the majority of the BrdU-positive cells consisted of fibrocytes, smooth muscle cells, and endothelial cells. Only 0.35%±0.26 of cardiac myocytes in the normotensive group showed positive BrdU-staining compared to 0.48%±0.32 in the hypertensive group. This difference was statistically not significant.
This study showed that early after onset of hypertension proliferation of non-myocytes, but not of myocytes occurred. DNA synthesis is limited almost completely to the interstitial cells and does not occur in any significant extent in cardiac myocytes. In conclusion, hyperplasia of cardiac myocytes is not observed at carly stages of hypertension, but it may develop at a late stage of cardiac adaptation.
Similar content being viewed by others
References
Anversa P, Capasso JM, Olivetti G, Sonnenblick EH (1992) Cellular basis of ventricular remodeling in hypertensive cardiomyopathy. Am J Hypertens 5: 758–770
Anversa P, Fitzpatrick D, Argani S, Capasso JM (1991) Myocyte mitotic division in the aging mammalian rat heart. Circ Res 69:1159–1164
Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Capasso JM (1990) Hypertensive cardiomyopathy. Myocyte nuclei hyperplasia in the mammalian rat heart. J Clin Invest 85:994–997
Baba HA, Takeda A, Nagano M (1995) The influence of decreased left-ventricular afterload on cardiac morphology in hypertrophied rat hearts. Int J Cardiol 49:107–117
Bishop SP (1973) Effect of aortic stenosis on myocardial cell growth, hyperplasia, and ultrastructure in neonatal dogs. Recent Adv Stud Cardiac Struct Metab 3:637–656
Claycomb WC (1975) Biochemical aspects of cardiac muscle differentiation. Deoxyribonucleic acid polymerase activity. J Biol Chem 250:3229–3235
Coppola JA, Coleb MB (1986) Constitutive c-myc oncogene expression blocks mouse erythroleukemia cell differentiation but not commitment. Nature 320: 760–763
Grove D, Nair KG, Zak R (1969) Biochemical correlates of cardiac hypertrophy. III. Change in DNA content; the relative contributions of polyploidy and mitotic activity. Circ Res 25:463–471
Grove D, Zak R, Nair KG, Aschenbrenner V (1969) Biochemical correlates of cardiac hypertrophy. VI. Observation on the cellular organisation of growth during myocardial hypertrophy in the rat. Circ Res 25:473–485
Hamada M, Nishio I, Baba A, Fukuda K, Takeda J, Ura M, Hano T, Kuchii M, Masuyama Y (1990) Enhanced DNA synthesis of cultured vascular smooth muscle cells from spontaneously hypertensive rats. Atherosclerosis 81:191–198
Klinge O, Stöcker E (1968) Die DNS-Synthese im Rattenherzen als Funktion des Lebensalters. Autoradiographische Untersuchung mit H3-Thymidin. Expertia 24:167–168
Komuro I, Kurabayashi M, Takaku F, Yazaki Y (1988) Expression of cellular oncogenes in the myocardium during the developmental stage and pressureoverload hypertrophy of the rat heart. Circ Res 62:1075–1079
Linzbach AJ (1960) Heart failure from the point of view of quantitative anatomy. Am J Cardiol 5:370–382
Liu Y, Cigola E, Cheng W, Kajstura J, Olivetti G, Hintze TH, Anversa P (1995) Myocyte nuclear mitotic division and cell death characterize the cardiac myopathy induced by rapid ventricular pacing in dogs. Lab Invest 73:771–787
Morkin E, Ashford TP (1968) Myocardial DNA synthesis in experimental cardiac hypertrophy. Am J Physiol 215: 1409–1413
Neffgen JF, Korecky B (1972) Cellular hyperplasia and hypertrophy in cardiomegalies induced by anemia in young and adult rats. Circ Res 30:104–113
Noguchi PD, Cunningham RE, Ridge J, Muller J (1985) The use of flow cytometry and cell sorting in a human colon carcinoma model. Cytometry 6: 254–259
Oberpriller JO, Ferrans VJ, Carroll RJ (1984) DNA synthesis in rat atrial myocytes as a response to left ventricular infarction. An autoradiographic study of enzymatically dissociated myocytes. J Mol Cell Cardiol 16:1119–1126
Pollack PS, Houser SR, Budjak R, Goldman B (1994) C-myc gene expression is localized to the myocyte following hemodynamic overload in vivo. J Cell Biochem 54:78–84
Rakusan K (1984) Cardiac growth, maturation and aging. In: Zak R (Ed) Growth of the Heart in Health and Disease. Raven Press, New York: 131–164
Reiss K, Capasso JM, Huang H-E, Meggs LG, Li P, Anversa P (1993) ANG II receptors, c-myc, and c-jun in myocytes after myocardial infarction and ventricular failure. Am J Physiol 264: H760-H769
Rumyantsev PP, Kassem AM (1976) Cumulative indices of DNA synthesizing myocytes in different compartments of the working myocardium and conductive system of the rats heart muscle following extensive left ventricle infarction. Virchows Arch B Cell Path 20:329–342
Tachibana H, Inoue S, Oki H, Andou H, Katagiri T (1993) Sequence in DNA synthetic phase ratio of spontaneous hypertensive rat (SHR) myocardium. Study on development of cardiomegaly. Jap Circ J 57:442–448
Vogel R, Spielmann H (1988) Cytotoxic and genotoxic effects of bromodeoxyuridine during in vitro labelling for sister-chromatid differentiation in preimplantation mouse embryos. Mutation Res 209:75–78
Zak R (1974) Development and proliferative capacity of cardiac muscle cells. Circ Res (Suppl II) 34/35:17–26
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Baba, H.A., Takeda, A., Schmid, C. et al. Early proliferative changes in hearts of hypertensive Goldblatt rats: an immunohistochemical and flow-cytometrical study. Basic Res Cardiol 91, 275–282 (1996). https://doi.org/10.1007/BF00789299
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00789299