Abstract
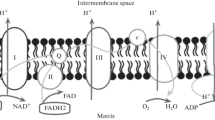
F1-ATPases are large multimeric proteins that can be isolated from the membrane bound system that catalyzes the phosphorylation of ADP by inorganic phosphate in bacteria, plants, and mitochondria. They can be visualized in electron micrographs of the inner mitochondrial membranes where they appear as large protruding spheres 90 Å in diameter. The purified F1-ATPases have a molecular weight of 320,000 to 400,000 daltons and are composed of five non-identical subunits (α, β, γ, δ and ε). The stoichiometry of these subunits in the complex is still unknown but compositions of the type α3β3γδε and α2β2γ2δ2ε2 were found to be consistent with some of the available experimental data. This review discusses the recent data and the experimental approaches utilized for the structural characterization of F1-ATPases.
Similar content being viewed by others
References
Amzel, L. M., and Pedersen, P. L. (1978).J. Biol. Chem. 253 2067–2069.
Amzel, L. M., and Pedersen, P. L. (1979).Enzymol. 55 333–337.
Baird, B. A., and Hammes, G. G. (1976).J. Biol. Chem. 251 6953–6962.
Baird, B. A., and Hammes, G.G. (1977).J. Biol. Chem. 252 4743–4748.
Bragg, P. D., and Hou, C. (1975).Arch. Biochem. Biophys. 167 311–321.
Cantley, L. C. Jr., and Hammes, G. H. (1975).Biochemistry 14 2976–2981.
antley, L. C., Jr., and Hammes, G. H. (1976).Biochemistry 15 1–8; 9–14.
Catterall, W. A., and Pedersen, P. L. (1971).J. Biol. Chem. 246 4987–4994.
Catterall, W. A., and Pedersen, P. L. (1973).J. Biol. Chem. 248 7427–7431.
Cohn, E. J., and Edsall, J. T. (1943).Proteins, Amino Acids and Peptides Reinhold, New York, p. 370.
Edelstein, S. J., and Schachman, H. K. (1967).J. Biol. Chem. 242 306–311.
Farron, F. (1970).Biochemistry 9 3823–3828.
Farron, F., and Racker, E. (1970).Biochemistry 9 3829–3836.
Fitzgerald, P. M. D., Stankiewicz, P. J., Campbell Smith, S., and McPhearson, A. (1979).J. Mol. Biol. 135 735–756.
Howell, S. H. and Moudrianakis, E. M. (1976).Proc. Natl. Acad. Sci. USA 58 1261–1268.
Kagawa, Y. (1978).Biochim. Biophys. Acta 505 45–93.
Kagawa, Y., and Racker, H.S. (1966).J. Biol. Chem. 241 2475–2482.
Kagawa, Y., Sone, N., Yoshida, M., Hirata, H., and Ikamoto, H. (1976).J. Biochem. 80 141–151.
Kawahara, K., and Tanford, C. (1966).Biochemistry 5 1578–1584.
Kielley, W. W., and Harrington, W. F. (1960).Biochim. Biophys. Acta 41 401.
Knowles, A. F., and Penefsky, H. S. (1972).J. Biol. Chem. 247 6624–6630.
Kozlov, I. A., and Skulachev, V. P. (1977).Biochim. Biophys. Acta 463 29–89.
Matsushima, M., Marquart, M., Jones, T. A., Colman, P. M., Bartels, K., Huber, R., and Palm, W. (1977).J. Mol. Biol. 121 441–59.
Mitchell, P. (1974).FEBS Let. 189–194.
Nelson, N.Biochim. Biophys. Acta 456, 314–338.
Paradies, H. H. (1979).Biochem. Biophys. Res. Commun. 91 685–692.
Paradies, H. H., and Schmidt, U. D. (1979).J. Biol. Chem. 254 5257–5263.
Paradies, H. H., Zimmermann, J., and Schmidt, U. D. (1978).J. Biol. Chem. 253 8972–8979.
Pedersen, P. L. (1975).J. Bioenerg. 6 243–275.
Pedersen, P. L., Amzel, L. M., Sopper, J. N., Cintron, N. and Hollihen, J. (1978).Proceedings of the Mosbach Colloquia on Energy Conservation in Biological Membranes Springer-Verlag, Berlin-New York (1978), pp. 159–194.
Penefsky, H. S. (1974). InThe Enzymes (Boyer, P. D., ed.), Vol. 10 Academic Press, New York, pp. 375–394.
Penefsky, H. S., and Warner, R. C. (1965).J. Biol. Chem. 240 4694–4702.
Reithel, F. J., and Sakura, J. D. (1963).J. Phys. Chem. 67 2497.
Schnebli, H. P., Vatter, A. E., and Abrams, A. (1970).J. Biol. Chem. 245 1122–1127.
Senior, A. E. (1975).Biochemistry 14 660–664.
Senior, A. E. (1978).Membrane Proteins in Energy Transduction (Capaldi, R. A., ed.). Marcel Dekker, New York.
Schmidt, U. D., and Paradies, H. (1977b).Biochem. Biophys. Res. Commun. 78 1043–1052.
Vogel, G., and Steinhart, R. (1976).Biochemistry 15 208–215.
Schmidt, U. D., and Paradies, H. (1977b).Biochem. Biophys. Res. Commun. 78 383–392.
Wakabayashi, T., Kubota, M., Yoshida, M., and Kagawa, Y. (1977).J. Mol. Biol. 117 515.
Yphantis, D. A. (1964)Biochemistry 3 297–317.
Yoshida, M., Sone, N., Hirata, H., Kagawa, Y., and Ui, N. (1979).J. Biol. Chem. 254 9525–9533.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Amzel, L.M. Structure of F1-ATPases. J Bioenerg Biomembr 13, 109–121 (1981). https://doi.org/10.1007/BF00763833
Issue Date:
DOI: https://doi.org/10.1007/BF00763833