Abstract
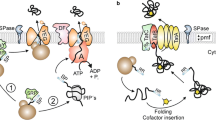
Numerous secretory proteins of the Gram-negative bacteriaE. coli are synthesized as precursor proteins which require an amino terminal extension known as the signal peptide for translocation across the cytoplasmic membrane. Following translocation, the signal peptide is proteolytically cleaved from the precursor to produce the mature exported protein. Signal peptides do not exhibit sequence homology, but invariably share common structural features: (1) The basic amino acid residues positioned at the amino terminus of the signal peptide are probably involved in precursor protein binding to the cytoplasmic membrane surface. (2) A stretch of 10 to 15 nonpolar amino acid residues form a hydrophobic core in the signal peptide which can insert into the lipid bilayer. (3) Small residues capable of β-turn formation are located at the cleavage site in the carboxyl terminus of the signal peptide. (4) Charge characteristics of the amino terminal region of the mature protein can also influence precursor protein export. A variety of mutations in each of the structurally distinct regions of the signal peptide have been constructedvia site-directed mutagenesis or isolated through genetic selection. These mutants have shed considerable light on the structure and function of the signal peptide and are reviewed here.
Similar content being viewed by others
References
Bankaitis, V. A., Rasmussen, B. A., and Bassford, P. J. (1984).Cell 37, 243–252.
Bankaitis, V. A., Altman, E., and Emr, S. D. (1987). InBacterial Outer Membranes as Model Systems (Inouye, M., ed.), Wiley, New York, pp. 75–116.
Batenburg, A. M., Brasseur, R., Ruysschaert, J., van Scharrenburg, G. J. M., Slotboom, A. J., Demel, R. A., and de Kruijff, B. (1988).J. Biol. Chem. 263, 4202–4207.
Bechwith, J., and Silhavy, T. (1984). InMethods in Enzymology (Fleischer, S., and Fleischer, B., eds.), Academic Press, New York, pp. 3–11.
Bedouelle, H., and Hofnay, M. (1981). InMembrane Transport and Neuroreceptors (Oxender, D.,et al., eds.), A. R. Liss, New York, pp. 399–403.
Bedouelle, H., Bassford, P. J., Fowler, A. V., Zabin, I., Beckwith, J., and Hofnung, M. (1980).Nature (London)285, 78–81.
Benson, S. A., Hall, M. N. and Rasmussen, B. A. (1987).J. Bacteriol. 169, 4686–4691.
Blobel, G., and Dobberstein, B. (1975).J. Cell. Biol. 67, 835–851.
Briggs, M. S., and Gierasch, L. M. (1986).Adv. Protein Chem. 38, 109–180.
Briggs, M. S., Gierasch, L. M., Zlotnick, A., Lear, J. D., and DeGrado, W. F. (1985).Science 228, 1096–1099.
Caulfield, M. R., Duong, L. T., Baker, R. K., Rosenblatt, M., and Lively, M. O. (1989).J. Biol. Chem. 264, 15813–15817.
Cavard, D., Baty, D., Howard, S. P., Verheij, H. M., and Lazdunski, C. (1987).J. Bacteriol. 169, 2187–2194.
Chen, L., Tai, P. C., Briggs, M. S., and Gierasch, L. M. (1987).J. Biol. Chem. 262, 1427–1429.
Chou, P. Y., and Fasman, G. D. (1978).Annu. Rev. Biochem. 47, 251–276.
Coleman, J., Inukai, M., and Inouye, M. (1985).Cell. 43, 351–360.
Collier, D. N., and Bassford, P. J. (1989).J. Bacteriol. 171, 4640–4647.
Cover, W. H., Ryan, J. P., Bassford, P. J., Walsh, K. A., Bollingen, J., and Randall, L. L. (1987).J. Bacteriol. 169, 1794–1800.
DeVrije, T., Batenburg, A. M., Jordi, W., and DeKruijff, B. (1989).Eur. J. Biochem. 180, 385–292.
Duffaud, G., and Inouye, M. (1988).J. Biol. Chem. 263, 10224–10228.
Emr, S. D., and Bassford, P. J. (1982).J. Biol. Chem. 257, 5852–5860.
Emr, S. D., and Silhavy, T. J. (1980).J. Mol. Biol. 141, 63–90.
Emr, S. D., and Silhavy, T. J. (1983).Proc. Natl. Acad. Sci. USA 80, 4599–4603.
Emr, S. D., Schwartz, M., and Silhavy, T. J. (1978).Proc. Natl. Acad. Sci. USA 75, 5802–5806.
Fikes, J. D., and Bassford, P. J. (1987).J. Bacteriol. 169, 2352–2359.
Fikes, J. D., Bankaitis, V. A., Ryan, J. P., and Bassford, P. J. (1987).J. Bacteriol. 169, 2345–2359.
Freudl, R., Braun, G., Hindennach, I., and Henning, U. (1985).Mol. Gen. Genet. 201, 76–81.
Freudl, R., MacIntyre, S., Degen, M., and Henning, U. (1988).J. Biol. Chem. 263, 344–349.
Garcia, PO. D., Ghrayeb, J., Inouye, M., and Walter, P. (1987).J. Biol. Chem. 262, 9463–9468.
Goldstein, J., Lehnhardt, S., and Inouye, M. (1990).J. Bacteriol. 172, 1225–1231.
Ghrayeb, J., Lunn, C. A., Inouye, S., and Inouye, M. (1985).J. Biol. Chem. 260, 10961–10965.
Hall, M. N., Gabay, J., and Schwartz, M. (1983).EMBO J. 2, 15–19.
Hartmann, E., Rapaport, T. A., and Lodish, H. F. (1989).Proc. Natl. Acad. Sci. USA 86, 5786–5790.
Iida, A., Groarke, J. M., Park, S., Thom, J., Zabicky, J. H., Hazelbauer, G. L., and Randall, L. L. (1985).EMBO J. 4, 1875–1880.
Iino, T., Takahashi, M., and Sako, T. (1987).J. Biol. Chem. 262, 7412–7417.
Inouye, M., and Halegoua, S. (1980).CRC Crit. Rev. Biochem. 7, 339–371.
Inouye, S., Wang, S., Sekizawa, J., Halegoua, S., and Inouye, M. (1977).Proc. Natl. Acad. Sci. USA 74, 1004–1008.
Inouye, S., Soberon, X., Franceschini, T., Nakamura, K., Itakura, K., and Inouye, M. (1982).Proc. Natl. Acad. Sci. USA 79, 3438–3441.
Inouye, S., Franceschini, T., Sato, M., Itakura, K., and Inouye, M. (1983a).EMBO J. 2, 87–91.
Inouye, S., Hsu, C-P. S., Itakura, K., and Inouye, M. (1983b).Science 221, 59–61.
Inouye, S., Vlasuk, G. P., Hsiung, H., and Inouye, M. (1984).J. Biol. Chem. 259, 3729–3733.
Inouye, S., Duffaud, G., and Inouye, M. (1986).J. Biol. Chem. 261, 10970–10975.
Kadonaga, J. T., Plueckthun, A., and Knowles, J. R. (1985).J. Biol. Chem. 260, 16192–16199.
Kendall, D. A., Bock, S. C., and Kaiser, E. T. (1986).Nature (London)321, 706–708.
Kuhn, A., and Wickner, W. (1985).J. Biol. Chem. 260, 15914–15918.
Lee, N., Yamagata, H., and Inouye, M. (1983).J. Bacteriol. 155, 407–411.
Lenhardt, S., Pollitt, S. and Inouye, M. (1987).J. Biol. Chem. 262, 1716–1719.
Lehnhardt, S., Pollitt, N. S., Goldstein, J., and Inouye, M. (1988).J. Biol. Chem. 263, 10300–10303.
Li, P., Beckwith, J., and Inouye, H. (1988).Proc. Natl. Acad. Sci. USA 85, 7685–7689.
Lin, J. J. C., Kanazuwa, H., Ozols, J., and Wu, H. (1978).Proc. Natl. Acad. Sci. USA 75, 4891–4895.
Lunn, C. A., and Inouye, M. (1987).J. Biol. Chem. 262, 8318–8324.
Michaelis, S., Inouye, H., Oliver, D., and Beckwith, J. (1983).J. Bacteriol. 154, 366–374.
Michaelis, S., Hunt, J. F., and Beckwith, J. (1986).J. Bacteriol. 167, 160–167.
Nikaido, H., and Wu, H. C. (1984).Proc. Natl. Acad. Sci. USA 81, 1048–1052.
Perlman, D., and Halvorson, H. O. (1983).J. Mol. Biol. 167, 392–409.
Pollitt, N. S., and Inouye, M. (1988).J. Bacteriol. 170, 2051–2055.
Pollitt, S., Inouye, S., and Inouye, M. (1985).J. Biol. Chem. 260, 7965–7969.
Pollitt, S., Inouye, S., and Inouye, M. (1986).J. Biol. Chem. 261, 1835–1837.
Pugsley, A. P. (1988). InProtein Transfer and Organelle Biogenesis (Das, R. C., and Robbins, P. W., eds.), Academic Press, New York, pp. 607–652.
Puziss, J. W., Fikes, J. D., and Bassford, P. J. (1989).J. Bacteriol. 171, 2303–2311.
Rasmussen, B. A., and Silhavy, T. J. (1987).Genes Dev. 1, 185–196.
Rousset, J. P., Gilson, E., and Hofnung, M. (1986).J. Mol. Biol. 191, 313–320.
Ryan, J. P., and Bassford, P. J. (1985).J. Biol. Chem. 260, 14832–14837.
Ryan, J. P., Duncan, M. C., Bankaitis, V. A., and Bassford, P. J. (1986).J. Biol. Chem. 261, 3389–3395.
Stader, J., Benson, S. A., and Silhavy, T. J. (1986).J. Biol. Chem. 261, 15075–15080.
Vlasuk, G. P., Inouye, S., Ito, H., Itakura, K., and Inouye, M. (1983).J. Biol. Chem. 258, 7141–7148.
Vlasuk, G. P., Inouye, S., and Inouye, M. (1984).J. Biol. Chem. 259, 6195–6200.
von Heijne, G. (1983).Eur. J. Biochem. 133, 17–21.
von Heijne, G. (1986a).Nucleic Acids Res. 14, 4683–4690.
von Heijne, G. (1986b).J. Mol. Biol. 192, 287–290.
Watson, M. E. E. (1984).Nucleic Acids Res. 12, 5145–5164.
Wickner, W. (1979).Annu. Rev. Biochem. 48, 23–45.
Wu, H. C. (1987). InBacterial Outer Membranes as Model Systems (Inouye, M., ed.), Wiley, New York, pp. 37–71.
Yamaguchi, K., Yu, F., and Inouye, M. (1988).Cell 53, 423–432.
Yamane, K., and Mizushima, S. (1988).J. Biol. Chem. 263, 19690–19696.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gennity, J., Goldstein, J. & Inouye, M. Signal peptide mutants ofEscherichia coli . J Bioenerg Biomembr 22, 233–269 (1990). https://doi.org/10.1007/BF00763167
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00763167