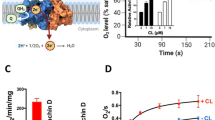
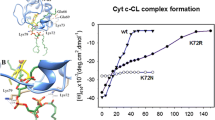
Abstract
Bovine cytochromec oxidase usually contains 3–4 mol of tightly bound cardiolipin per cytochromeaa 3 complex. At least two of these cardiolipins are required for full electron transport activity. Without the tightly bound cardiolipin, cytochromec oxidase has only 40–50% of its original activity when assayed in detergents that support activity, e.g., dodecyl maltoside. By measuring the restoration of electron transport activity, functional binding constants for cardiolipin and a number of cardiolipin analogues have been evaluated (K d,app=1 µM for cardiolipin). These binding constants agree reasonably well with direct measurement of the binding using [14C]-acetyl-cardiolipin (K d <0.1 µM) when the enzyme is solubilized with Triton X-100. These data are discussed in relationship to the wealth of data that is known about the association of cardiolipin with cytochromec oxidase and the other mitochrondrial electron transport complexes and transporters.
Similar content being viewed by others
References
Adramovitch, D. A., Marsh, D., and Powell, G. L. (1990).Biochim. Biophys. Acta 1020 34–42.
Al-Tai, W. F., Jones, M. G., Rashid, K., and Wilson, M. T. (1983).Biochem. J. 209 901–903.
Al-Tai, W. F., Jones, M. G., and Wilson, M. T. (1984).Comp. Biochem. Physiol. 77B 609–616.
Awasthi, Y. C., Chuang, T. F., Kennan, T. W., and Crane, F. L. (1971).Biochim. Biophys. Acta 226 42–52.
Beleznai, Z., and Jancsik (1989).Biochem. Biophys. Res. Commun. 159 132–139.
Beyer, K., and Klingenberg, M. (1985).Biochemistry 15 3821–3826.
Bligh, E. G., and Dyer, W. J. (1959).Can. J. Biochem. Physiol. 37 911–917.
Brandolin, G., Doussiere, J., Gulik, A., Gulik-Krzywicki, T., Lauquin, G. J. M., and Vignais, P. V. (1980).Biochim. Biophys. Acta 592 592–614.
Brierley, G. P., and Merola, A. J. (1962).Biochim. Biophys. Acta 64 205–217.
Cable, M. B., and Powell, G. L. (1980).Biochemistry 19 5679–5686.
cheneval, D., and Carafoli, E. (1988).Eur. J. Biochem. 171 1–9.
Cheneval, D., Muller, M., and Carafoli, E. (1983).FEBS Lett. 159 123–126.
Cheneval, D., Muller, M., Toni, R., Ruetz, S., and Carafoli, E. (1985).J. Biol. Chem. 260 13003–13007.
Dale, M. P., and Robinson, N. C. (1988a).Biochemistry 27 8270–8275.
Dale, M., and Robinson, N. C. (1988b).FASEB J. 2, A774.
Demant, E. J. F. (1983).Eur. J. Biochem. 137 113–118.
Demant, E. J. F., and Jensen, P. K. (1983).Eur. J. Biochem. 132 551–556.
Demel, R. A., Jordi, W., Lambrechts, H., van Damme, H., Hovius, R., and de Kruijff, B. (1989).J. Biol. Chem. 264 3988–3997.
Eilers, M., Endo, T., and Schatz, G. (1989).J. Biol. Chem. 264 2945–2950.
Fowler, W. T., Lambeth, J. D., and Powell, G. L. (1988).Chem. Phys. Lipids 47 261–271.
Fry, M., and Green, D. E. (1980).Biochem. Biophys. Res. Commun. 93 1238–1245.
Fry, M., and Green, D. E. (1981).J. Biol. Chem. 256 1874–1880.
Fry, M., Blondin, G. A., and Green, D. E. (1980).J. Biol. Chem. 255 9967–9970.
Goormaghtigh, E., and Ruysschaert, J. (1984).Biochim. Biophys. Acta 779 271–288.
Goormaghtigh, E., Chatelain, P., Caspers, J., and Ruysschaert, J. M. (1980).Biochim. Biophys. Acta 597 1–14.
Goormaghtigh, E., Brasseur, R., and Ruysschaert, J. (1982).Biochem. Biophys. Res. Commun. 104 314–320.
Goormaghtigh, E., Huart, P., Brasseur, R., and Ruysschaert, J.-M. (1986).Biochim. Biophys. Acta 861 83–94.
Hasinoff, B. B., and Davey, J. P. (1988).Biochem. J. 250 827–834.
Hill, B. C., Cook, K., and Robinson, N. C. (1988).Biochemistry 27 4741–4747.
Hoch, F. L. (1992).Biochim. Biophys. Acta 1113 71–133.
Ioannou, P. V., and Golding, B. T. (1979).J. Lipid Res. 17 279–318.
Kadenbach, B., Mende, P., Kolbe, H. V. J., Stipani, I., and Palmieri, F. (1982).FEBS Lett. 139 109–112.
Kadenbach, B., Jarausch, J., Hartmann, R., and Merle, P. (1983).Anal. Biochem.,129 517–521.
Kaplan, R. S., Pratt, R. D., and Pedersen, P. L. (1986).J. Biol. Chem. 261 12767–73.
Knowles, P. F., Watts, A., and Marsh, D. (1981).Biochemistry 20 5888–5894.
Kramer, R., and Klingenberg, M. (1980).FEBS Lett. 119 257–260.
Kuppe, A., Mrsny, R. J., Shimizu, M., Firsan, S. J., Keana, J. F. W., and Griffith, O. H. (1987).Biochemistry 26 7693–7701.
Mahapatro, S. N., and Robinson, N. C. (1990).Biochemistry 29 764–770.
Marsh, D., and Powell, G. L. (1988).Bioelectrochem. Bioenerg.,20 73–82.
Mende, P., Kolbe, H. V. J., Kadenbach, B., Stipani, I., and Palmieri, F. (1982).Eur. J. Biochem.,128 91–95.
Mende, P., Huther, J.-J., and Kadenbach, B. (1983).FEBS Lett. 158 331–334.
Muller, M., Moser, R., Cheneval, D., and Carafoli, E. (1985).J. Biol. Chem.,260 3839–3843.
Myers, M., Mayorga, O., Emtage, J., and Freire, E. (1987).Biochemistry 26 4309–4315.
Nalecz, K. A., Bolli, R., Wojtczak, L., and Azzi, A. (1986).Biochim. Biophys. Acta 851 29–37.
Nicolay, K., and deKruijff, B. (1987).Biochim. Biophys. Acta 892 320–330.
Nicolay, K., Timmers, R. J. M., Spoelstra, E., van der Neut, R., Fok, J. J., Huigen, Y., Verkleij, A. J., and de Kruijff, B. (1984).Biochim. Biophys. Acta 778 359–371.
Nishijima, S., Asami, Y., Uetake, N., Yamagoe, S., Ohta, A., and Shibuya, I. (1988).J. Bacteriol. 170 775–780.
Noel, H., and Pande, S. V. (1986).Eur. J. Biochem. 155 99–102.
Ortega-Lopez, J., and Robinson, N. C. (1993). unpublished data.
Ou, W.-J., Ito, A., Umeda, M., Inoue, K., and Omura, T. (1988).J. Biochem.,103 589–595.
Powell, G. L., Knowles, P. F., and Marsh, D. (1985).Biochim. Biophys. Acta 816 191–194.
Powell, G. L., Knowles, P. F., and Marsh, D. (1987).Biochemistry 26 8138–8145.
Robinson, N. C. (1982).Biochemistry 21 184–188.
Robinson, N. C. (1990).J. Lipid Res. 31 1513–1516.
Robinson, N. C., and Capaldi, R. A. (1977).Biochemistry 16 375–380.
Robinson, N. C., and Wiginton, D. (1985).J. Inorg. Biochem.,23 171–176.
Robinson, N. C., Strey, F., and Talbert, L. (1980).Biochemistry 19 3656–3661.
Robinson, N. C., Zborowski, J., and Talbert, L. H. (1990).Biochemistry 29 8962–8969.
Speck, S. H., Neu, C. A., Swanson, M. S., and Margoliash, E. (1983).FEBS Lett. 164 379–382.
Tamm, L. K. (1986).Biochemistry 25 7470–7476.
Thompson, D. A., and Ferguson-Miller, S. (1983).Biochemistry 22 3178–3187.
Vik, S. B., Georgevich, G., and Capaldi, R. A. (1981).Proc. Natl. Acad. Sci. USA 78 1456–1460.
Watts, A., Marsh, D., and Knowles, P. F. (1978).Biochem. Biophys. Res. Commun. 81 403–409.
Yu, C., Yu, L., and King, T. E. (1975).J. Biol. Chem. 250 1383–1392.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robinson, N.C. Functional binding of cardiolipin to cytochromec oxidase. J Bioenerg Biomembr 25, 153–163 (1993). https://doi.org/10.1007/BF00762857
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00762857