Summary
-
1.
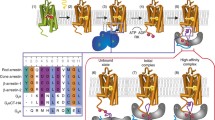
The visual transduction system of the vertebrate retina is a well-studied model for biochemical and molecular studies of signal transduction. The structure and function of rhodopsin, a prototypical G protein-coupled receptor, and transducin or G t , the photoreceptor G protein, have been particularly well studied. Mechanisms of rhodopsin-G t interaction are discussed in this review.
-
2.
The visual pigment rhodopsin contains a chromophore, and thus conformational changes leading to activation can be monitored spectroscopically. A model of the conformational changes in the activated receptor is presented based on biophysical and biochemical data.
-
3.
The current information on sites of interaction on receptors and cognate G proteins is summarized. Studies usng synthetic peptides from amino acid sequences corresponding to G t and rhodopsin have provided information on the sites of rhodopsin-G t interaction. Synthetic peptides from the carboxyl terminal region ofα t mimic G t by stabilizing the active conformation of rhodopsin, Metarhodopsin II.
-
4.
The conformation of one such peptide when it is bound to Metarhodopsin II was determined by 2D NMR. The model based on the NMR data was tested using peptide analogs predicted to stabilize or break the structure. These studies yield molecular insight into why toxin-treated and mutant G proteins are uncoupled from receptors.
Similar content being viewed by others
References
Aoki, C., Zemcik, B. A., Strader, C. D., and Pickel, V. M. (1989). Cytoplasmic loop ofβ-adrenergic receptors: Synaptic and intracellular localization, and relation to catecholaminergic neurons in the nuclei of the solitary tracts.Brain Res. 493331–347.
Baehr, W., and Applebury, M. L. (1986). Exploring visual transduction with recombinant DNA techniques.Trends Neuro. Sci. 9198–203.
Bennett, N., and Dupont, Y. (1985). The G-protein of retinal rod outer segments (transducin). Mechanism of interaction with rhodopsin and nucleotides.J. Biol. Chem. 2604156.
Bennett, N., Michael-Villaz, M., and Kühn, H. (1982). Light-induced interaction between rhodopsin and the GTP-binding protein. Metarhodopsin II is the major photoproduct involved.Eur. J. Biochem. 12797–103.
Chabre, M., and Deterre, P. (1989). Molecular mechanism of visual transduction.Eur. J. Biochem. 179255–266.
Cote, T. E., Frey, E. A. and Sekura, R. D. (1984). Altered activity of the inhibitory guanyl nucleotide-binding component (Ni) induced by pertussis toxin.J. Biol. Chem. 2598693–8698.
Cotecchia, S., Exum, S., Caron, M. G., and Lefkowitz, R. J. (1990). Regions of theα 1-adrenergic receptor involved in coupling to phosphatidylinositol hydrolysis and enhanced sensitivity of biological function.Proc. Natl. Acad. Sci. 872896–2900.
Deretic, D., and Hamm, H. E. (1987). Topographic analysis of antigenic determinants recognized by monoclonal antibodies to the photoreceptor guanyl nucleotide-binding protein, transducin.J. Biol. Chem. 26210831–10847.
Dixon, R. A. F., Sigal, I. S., Rands, E., Register, R. B., Candelore, M. R., Blake, A. D., and Strader, C. D. (1987). Ligand binding to theβ-adrenergic receptor involves its rhodopsin-like core.Nature 32673–77.
Dixon, R. A. F., Sigal, I. S., and Strader, C. D. (1988). Structure-function analysis of theβ-adrenergic receptor.Cold Spring Harbor Symp. Quant. Biol. 53487–497.
Dohlman, H. G., Bouvier, M., Benovic, J. L, Caron, M. G., and Lefkowitz, R. J. (1987). The multiple membrane spanning topography of theβ 2-adrenergic receptor.J. Biol. Chem. 26214282–14288.
Dratz, E. A., and Hargrave, P. A. (1983). The structure of rhodopsin and the rod outer segment disk membrane.Trends Biochem. Sci. 8128–131.
Dratz, E. A., Furstenau, J. F., Hamm, H. E., and Hargrave, P. A. (1990). Probing the molecular mechanism of visual excitation using bioactive peptide sequences.Invest. Ophthalmol. Vis. Sci. 31(4):Suppl. 79.
Dratz, E. A., Hamm, H. E., Zwolinski, R., Furstenau, J., and Lambert, C. (1991). The 3-D structure of the active interface between rhodopsin and G protein determined using 2D-NMR.Biophys. J. 59:189a.
Emeis, D., Kühn, H., Reichert, J., and Hofmann, K. P. (1982). Complex formation between metarhodopsin II and GTP-binding protein in bovine photoreceptor membranes leads to a shift of the photoproduct equilibrium.FEBS. Lett. 14329–34.
Findlay, J. B. C. (1986). The structure of rhodopsin.Photobichem. Photobiophys. 13213–228.
Findlay, J. B. C., and Pappin, D. J. C. (1986). The opsin family of proteins.Biochem. J. 238625–642.
Franke, R. R., Sakmar, T. P., Oprian, D. D., and Khorana, H. C. (1988). A single amino acid substitution in rhodopsin (Lys248-Leucine) prevents activation of transducin.J. Biol. Chem. 2632119–2122.
Fraser, C. M., Chung, F.-Z., Wang, C.-D., and Venter, J. C. (1988). Site-directed mutagenesis of humanβ-adrenergic receptors: substitution of aspartic acid-130 by asparagine produces a receptor with high-affinity agonist binding that is uncoupled from adenylate cyclase.Proc. Natl. Acad. Sci. 855478–5482.
Fukada, Y., Takao, T., Ohguro, H., Yoshizawa, T., Akino, T., and Shimonishi, Y. (1990). Farnesylatedγ subunit of photoreceptor G protein indispensable for GTP binding.Nature 346658–660.
Furstenau, J. E., Starkey, J. A., Hamm, H. E., Hargrave, P. A. and Dratz, E. A. (1990). Studies of biologically active protein fragment conformations using 2D-NMR methods.Biophys. J. 57:43a.
Gilman, A. G. (1987). G proteins: Transducers of receptor-generated signals.Annu. Rev. Biochem. 56615–649.
Halpern, J. L., and Moss, J. (1990). Immunological characterization of guanine nucleotide-binding proteins: Effects of a monoclonal antibody against theγ subunit of transducin on guanine nucleotide-binding protein-receptor interactions.Mol. Pharm. 37797–800.
Hamm, H. E. (1990). Surfaces of interaction between G t and rhodopsin in the GDP-bound and empty-pocket configurations. In Robinson, A., and Greengard, P. (ed.),Advances in Second Messenger and Phosphoprotein Research, Raven Press, N.Y. Vol. 24, pp. 76–82.
Hamm, H. E., and Bownds, M. D. (1984). A monocolonal antibody to guanine nucleotide binding protein inhibits the light-activated cyclic GMP pathway in frog rod outer segments.J. Gen. Physiol. 84265–280.
Hamm, H. E., and Bownds, M. D. (1986). The protein complement of rod outer segments of the frog retina.Biochemistry 254512–4523.
Hamm, H. E., Deretic, D., Hofmann, K. P., Schleicher, A., and Kohl, B. (1987). Mechanism of action of monoclonal antibodies that block the light activation of the guanyl nucleotide-binding protein, transducin.J. Biol. Chem. 26210831–10838.
Hamm, H. E., Deretic, D., Arendt, A., Hargrave, P. A., Koenig, B., and Hofmann, K. P. (1988). Site of G protein binding to rhodopsin mapped with synthetic peptides from theα subunit.Science 241832–835.
Hamm, H. E., Zwolinski, R., Rarick, H. M., Furstenau, J., and Dratz, E. A. (1991). Probing the structure of the rhodopsin binding domain of rod G protein.Biophys. J. 59:374a.
Henderson, R., and Unwin, P. N. T. (1975). Three-dimensional model of purple membrane obtained by electron microscopy.Nature 25728–32.
Henderson, R., Baldwin, J. M., Ceska, T. K., Zemlin, F., Beckmann, E., and Downing, K. H. (1990). Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy.J. Mol. Biol. 213899–929.
Higashijima, T., Burnier, J., and Ross, E. M. (1990). Regulation of G i and G o by mastoparan, related amphiphilic peptides and hydrophobic amines: Mechanism and structural determinants of activity.J. Biol. Chem. 26514176–14186.
Hofmann, K. P. (1986). Photoproducts of rhodopsin in the disc membrane.Photobiochem. Photobiophys. 13309–327.
Kelleher, D. J., and Johnson, G. L. (1988). Transducin inhibition of light-dependent rhodopsin phosphorylation: Evidence forβγ subunit interaction with rhodopsin.Mol. Pharm. 34452–460.
Kobilka, B. K., Kobilka, T. S., Regan, J. W., Caron, M. G., and Lefkowitz, R. J. (1988). Chimericα 2-β 2-adrenergic receptors: delineation of domains involved in effector coupling and ligand binding specificity.Science 2401310–1315.
Koenig, B., Arendt, A., McDowell, J. H., Kahlert, M., Hargrave, P. A., and Hofmann, K. P. (1989). Three cytoplasmic loops of rhodopsin interact with transducin.Proc. Natl. Acad. Sci. USA 866878–6882.
Kubo, T., Bujo, H., Akiba, I., Nakai, J., Mishina, M., and Numa, S. (1988). Location of a region of the muscarinic acetylcholine receptor involved in selective effector coupling.FEBS Lett. 241119–125.
Kühn, H. (1981). Interactions of rod cell proteins with the disk membrane: Influence of light, ionic strength, and nucleotides.Curr. Top. Membr. Transp. 15171–201.
Kühn, H., and Hargrave, P. H. (1981). Light-induced binding of guanosine triphosphase to bovine photoreceptor membranes: Effect of limited proteolysis of the membranes.Biochemistry 202410–2417.
Kurose, H., Katada, T., Amano, T., and Ui, M. (1983). Specific uncoupling by islet-activating protein, pertussis toxin, of negative signal transduction viaα-adrenergic, cholinergic, and opiate receptors in neuroblastoma × glioma hybrid cells.J. Biol. Chem. 2584870.
Lamola, A. A., Yamane, T., and Zipp, A. (1974). Effects of detergents and high pressures upon the metarhodopsin I metarhodopsin II equilibrium.Biochemistry 15738–745.
Lazarevic, M., Rasenick, M. M., and Hamm, H. E. (1990). Modulation of adenylyl cyclase activity by G protein specific synthetic peptides in C6 glioma cells.Soc. Neurosci. Abstr. 16.
Liebman, P. A., Parker, K. R., and Dratz, E. A. (1987). The molecular mechanism of visual excitation and its relation to the structure and composition of the rod outer segment.Annu. Rev. Physiol. 49765–791.
Matthews, R. G., Hubbard, R., Brown, P. K., and Wald, G. (1963). Tautomeric forms of metarhodopsin.J. Gen. Physiol. 47215–240.
Mazzoni, M. R., and Hamm, H. E. (1989). Effects of monocolonal antibody binding on subunit interactions of the rod outer segment G protein, G t .Biochemistry 289873–9880.
Michel-Villaz, M., Saibil, H., and Chabre, M. (1979). Orientation of rhodopsinα-helices in retinal rod outer segment membranes studied by infrared linear dichroism.Proc. Natl. Acad. Sci. USA 764405–4408.
O'Dowd, B. F., Hanatowich, M., Regan, J. W., Leader, W. M., Caron, M. G., and Lefkowitz, R. J. (1988). Site-directed mutagenesis of the cytoplasmic domains of the humanβ 2-adrenergic receptor.J. Biol. Chem. 26315985–15992.
O'Dowd, B. F., Hnatowich, M., Caron, M. G., and Lefkowitz, R. J. (1989). Palmitoylation of the humanβ 2-adrenergic receptor.J. Biol. Chem. 2647564–7569.
Ovchinnikov, Y. A. (1987). Probing the folding of membrane proteins.Trends Biochem. Sci. 12434–438.
Ovchinnikov, Y. A., Abdulaev, N. G., and Bogachuk, A. S. (1988). Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated.FEBS Lett. 2301–5.
Ross, E. M. (1989). Signal sorting and amplification through G protein-coupled receptors.Neuron 3141–152.
Rubenstein, R. C., Wong, S. K-F., and Ross, E. M. (1987). The hydrophobic tryptic core of theβ-adrenergic receptor retains G s regulatory activity in response to agonists and thiols.J. Biol. Chem. 26216655–16662.
Schleicher, A., and Hofmann, K.-P. (1987). Kinetic study on the equilibrium between membrane-bound and free photoreceptor G-protein.J. Membrane Biol. 95271–281.
Shapiro, R. A., and Nathanson, N. M. (1989). Deletion analysis of the mouse M1 muscarinic Acetylcholine receptor: effects on phosphoinositide metabolism and down-regulation.Biochemistry 288946–8950.
Shichi, H., and Shelton, E. (1974). Assessment of physiological integrity of sonicated retinal rod membranes.J. Supramol. Struct. 27–17.
Sternweis, P. C. (1986). The purifiedα subunits of G o and G i from bovine brain requireβγ for association with phospholipid vesicles.J. Biol. Chem. 261631–637.
Strader, C. D., Dixon, R. A. F., Cheung, A. H., Candelore, M. R., Blaker, A. D., and Sigal, I. S. (1987). Mutations that uncouple theβ-adrenergic receptor from G s and increase agonist affinity.J. Biol. Chem. 26216439–16443.
Strader, C. D., Sigal, I. S., and Dixon, R. A. F. (1989). Structural basis ofβ-adrenergic receptor function.FASEB J. 31825–1832.
Sullivan, K. A., Miller, R. T., Masters, S. B., Beiderman, B., Heideman, W., and Bourne, H. R. (1987). Identification of receptor contact site involved in receptor-G protein coupling.Nature (Lond.)330758–762.
Vaillancourt, R. R., Dhanasekaran, N., Johnson, G. L., and Ruoho, A. E. (1990). 2-Azido-[32P]NAD+, a photoactivatable probe for G-protein structure: evidence for holotransducin oligomers in which the ADP-ribosylated carboxyl terminus ofα interacts with bothα andγ subunits.Proc. Natl. Acad. Sci. 873645–3649.
Vuong, T. M., Chabre, M., and Stryer, L. (1984). Millisecond activation of transducin in the cyclic nucleotide cascade of vision.Nature (Lond.)311659–661.
Wess, J., Brann, M. R., and Bonner, T. I. (1989). Identification of a small intracullar region of the muscarinic m3 receptor as a determinant of selective coupling to PI turnover.FEBS Lett. 258133–136.
Wilden, U., Hall, S. W., and Kühn, H. (1986). Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments.Proc. Natl. Acad. Sci. USA 831174–1178.
Wistow, G. J., Katial, A., Craft, C., and Shinohara, T. (1986). Sequence analysis of bovine retinal S-antigen. Relationships withα-transducin and G-proteins.FEBS Lett. 19623–27.
Wong, S. K.-F., Parker, E. M., and Ross, E. M. (1990). Chimeric muscarinic cholinergic:β-adrenergic receptors that activate G s in response to muscarinic agonists.J. Biol. Chem. 2656219–6224.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hamm, H.E. Molecular interactions between the photoreceptor G protein and rhodopsin. Cell Mol Neurobiol 11, 563–578 (1991). https://doi.org/10.1007/BF00741446
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00741446