Abstract
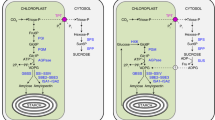
The rates of incorporation of various metabolites into starch by isolated amyloplasts from developing endosperm of spring wheat (Triticum aestivum L. cv. Axona) were examined. Of the metabolites tested that were likely to be present in the cytosol at concentrations sufficient to sustain starch synthesis, only glucose 1-phosphate (Glc1P) supported physiologically relevant rates of starch synthesis. Incorporation of Glc1P into starch was both dependent on the presence of ATP and intact organelles. The rate of incorporation of hexose into starch became saturated at a Glc1P concentration of less than 1 mol·m-3 in the presence of 1 mol·m-3 ATP. Starch synthesis from 5 mol · m-3 ADP-glucose supplied to the organelles occurred at rates 15-fold higher than from similar concentrations of Glc1P, but it is argued that this is probably of little physiological relevance. The net incorporation of hexose units into starch from GlclP was inhibited 50% by 100 mmol.m-3 carboxyatractyloside. Carbohydrate oxidation in the amyloplast was stimulated by the addition of 2-oxoglutarate and glutamine, and in such circumstances incorporation of14C-labelled metabolites into starch was reduced. Glucose 6-phosphate proved to be a better substrate for oxidative pathways than Glc1P. Our results suggest that Glc1P is the primary substrate for starch synthesis in developing wheat endosperm, and that ATP required for starch synthesis is imported via an adenylate translocator.
Similar content being viewed by others
Abbreviations
- ADP-glucose:
-
adenosine-5′diphosphoglucose
- APPase:
-
alkaline pyrophosphatase
- CAT:
-
carboxyatractyloside
- DHAP:
-
dihydroxyacetone phosphate
- Glc1P:
-
glucose 1-phosphate
- Glc6P:
-
glucose 6-phosphate
- OPPP:
-
oxidative pentose phosphate pathway
References
Bowsher, C.G., Boulton, E.L., Rose, J., Nayagam, S., Emes, M.J. (1992) Reductant for glutamate synthase is generated by the oxidative pentose phosphate pathway in non-photosynthetic root plastids. Plant J. 2, 893–898
Borchert, S., Grosse, H., Heldt, H.W. (1989) Specific transport of inorganic phosphate, glucose 6-phosphate, dihydroxyacetone phosphate and 3-phosphoglycerate into amyloplasts from pea roots. FEBS Lett. 253, 183–186
Borchert, S., Harborth, J., Schünemann, D., Hoferichter, P., Heldt, H.W. (1993) Studies of the enzymic capacities and transport properties of pea root plastids. Plant Physiol. 101, 303–312
Dennis, D.T., Miernyk, J.A. (1982) Compartmentation of non-photosynthetic carbohydrate metabolism. Annu. Rev. Plant. Physiol. 33, 27–50
Emes, M.J., Traska, A. (1987) Uptake of inorganic phosphate by plastids purified from the roots of isum sativum L. J. Exp. Bot. 38, 1781–1788
Entwistle, G., ap Rees, T. (1988) Enzymic capacities of amyloplasts from wheat (Triticum aestivum) endosperm. Biochem. J. 255, 391–396
Hatzfeld, W.-D., Stitt, M. (1990) A study of the rate of recycling of triose phosphates in heterotrophicChenopodium rubrum cells, potato tubers, and maize endosperm. Planta180, 198–204
Hill, L., Smith, A.M. (1991) Evidence that glucose 6-phosphate is imported as the substrate for starch synthesis by the plastids of developing pea embryos. Planta185, 91–96
Journet, E.P., Douce, R. (1985) Enzymic capacities of purified cauliflower bud plastids for lipid synthesis and carbohydrate metabolism. Plant Physiol. 79, 458–467
Keeling, P.L., Wood, J.R., Tyson, R.H., Bridges, I.G. (1988) Starch biosynthesis in developing wheat grain. Plant Physiol. 87, 311–319
King, J. (1970) Phosphoglucomutase. In: Methoden der enzymatischen Katalyse, pp. 764–785, Bergmeyer, H.U., ed. Verlag Chemie, Weinheim, FRG
Klingenberg, M., Heldt, H.W. (1982) The ADP/ATP translocator in mitochondria and its role in intracellular compartmentation. In: Metabolic compartmentation, pp. 101–120, Sies I., ed. Academic Press, New York
Lin, T.-P., Caspar, T., Somerville, C., Preiss, J. (1988) A starch-deficient mutant of Arabidopsis thaliana with low ADP-glucose pyrophosphorylase activity lacks one of the two subunits of the enzyme. Plant Physiol. 88, 1175–1181
Menzies, I.S., Seakins, J.W.T. (1976) Sugars. In: Chromatographic and electrophoretic techniques, 4th edn. Vol. 1. pp. 183–217, Smith, I., Seakins, J.W.T. eds. Heinemann, London
Müller-Rober, B., Sonnewald, U., Willmitzer, L. (1992) Inhibition of the ADP-glucose pyrophosphorylase in transgenic potatoes leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J. 11, 1229–1238
Ngernprasirtsiri, J., Takabe, T., Akazawa, T. (1989) Immunochemical analysis shows that an ATP/ADP-translocator is associated with the inner-envelope membranes of amyloplasts fromAcer pseudoplatanus L. Plant Physiol. 89, 1024–1027
Perata, P., Pozueta-Romero, J., Yamaguchi, J., Akazawa, T. (1992) Artefactual detection of ADP-dependent sucrose synthase in plant crude extracts. FEBS Lett309, 283–287
Pozueta-Romero, J., Frehner, M., Viale, A.M., Akazawa, T. (1991a) Direct transport of ADP-glucose by an adenylate translocator is linked to starch biosynthesis in amyloplasts. Proc. Natl. Acad. Sci. USA88, 5769–5773
Pozueta-Romero, J., Yamaguchi, J., Akazawa, T. (1991b) ADPG formation by the ADP-specific cleavage of sucrose — reassessment of sucrose synthase. FEBS Lett. 291, 233–237
Smith, A.M. (1990) Enzymes of starch synthesis. In: Methods in plant biochemistry, vol 3: Enzymes of primary metabolism, pp. 93–102, Lea, P.J., ed. Academic Press, London
Tetlow, I.J., Blissett, K.J., Emes, M.J. (1993) A rapid method for the isolation of purified amyloplasts from wheat endosperm. Planta189, 597–600
Tyson, R.H., ap Rees, T. (1988) Starch synthesis by isolated amyloplasts from wheat endosperm. Planta175, 33–38
Viola, R., Davies, H.V., Chudeck, A.R. (1991) Pathways of starch and sucrose biosynthesis in developing tubers of potato (Solanum tuberosum L.) and seeds of faba bean (Vicia faba L.). Planta183, 202–208
Author information
Authors and Affiliations
Additional information
The authors gratefully acknowledge the financial support of the Science and Engineering Research Council and helpful discussions with Professor Tom ap Rees, Department of Plant Sciences (Cambridge) and Dr. Lionel Hill (John Innes Institute, Norwich, UK). We also thank Mr. T.W. Heaton for growing the wheat at the Experimental Grounds and Miss L.C. Monks for typing the manuscript.
Rights and permissions
About this article
Cite this article
Tetlow, I.J., Blissett, K.J. & Emes, M.J. Starch synthesis and carbohydrate oxidation in amyloplasts from developing wheat endosperm. Planta 194, 454–460 (1994). https://doi.org/10.1007/BF00714456
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00714456