Summary
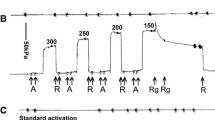
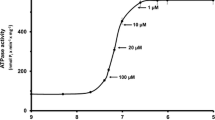
Rigor complexes between actin and myosin have been shown to cause increased binding of Ca2+ to troponin C. A similar effect of force-generating crossbridges has been suggested as an explanation for the coupling between load and activation which has been observed in skeletal and cardiac muscle. The goal of this study was to test the hypothesis that Ca2+-troponin affinity during crossbridge cycling is load-dependent. Ca2+-binding to detergent-extracted rabbit psoas fibres was measured during ATP-induced force generation and in the relaxed state. To compare Ca2+ binding in the latter two states it was necessary to establish conditions in which ATP-induced force could be regulated independently of free Ca2+ concentration. Such conditions were obtained by the use of either the ATPase inhibitor sodium vanadate or the substitution of MgITP for MgATP as an energy source. This study showed that in the presence of MgATP (or MgITP) the amount of Ca2+ bound to the myofilaments at a given free Ca2+ concentration was independent of the force generated. Thus forceper se is not a determinant of Ca2+-troponin affinity.
Similar content being viewed by others
References
ALLEN, D. G. & KURIHARA, S. (1982) The effects of muscle length on intracellular calcium transients in mammalian cardiac muscle.J. Physiol. 327 79–94.
ASHLEY, C. C. & MOISESCU, D. G. (1977) Effect of changing the composition of the bathing solutions upon the isometric tension-pCa relationship in bundles of crustacean myofibrils.J. Physiol. 270 627–52.
BERS, D. M. (1982) A simple method for the accurate determination of [Ca2+] in Ca-EGTA solutions.Am. J. Physiol. 242 C404–8.
BREMEL, R. D. & WEBER, A. (1972) Cooperation within actin filaments in vertebrate skeletal muscle.Nature, New Biol. 238 97–101.
DONALDSON, S. K. B. & KERRICK, W. G. L. (1975) Characterization of the effects of Mg2+ on Ca2+- and Sr2+-activated tension generation of skinned skeletal muscle fibers.J. gen. Physiol. 66 427–44.
EBASHI, S., KODAMA, A. & EBASHI, F. (1968) Troponin. I. Preparation and physiological function.J. Biochem. 64 465–77.
EDMAN, K. A. P. (1975) Mechanical deactivation induced by active shortening in isolated muscle fibres of the frog.J. Physiol. 246 255–75.
EDMAN, K. A. P. & KEISSLING, A. (1971) The time course of the active state in relation to sarcomere length and movement studied in single skeletal muscle fibers of the frog.Acta physiol. scand. 81 182–96.
EKELUND, M. C. & EDMAN, K. A. P. (1982) Shortening-induced deactivation of skinned fibres of frog and mouse striated muscle.Acta physiol. scand. 116 189–99.
ENDO, M. (1972) Stretch-induced increase in activation of skinned muscle fibres by calcium.Nature, New Biol. 237 211–13.
FABIATO, A. & FABIATO, F. (1978) Effects of pH on the myofilaments and sarcoplasmic reticulum of skinned cells from cardiac and skeletal muscle.J. Physiol. 276 233–55.
FABIATO, A. & FABIATO, F. (1979) Calculator programs for computing the composition of the multiple metals and ligands used for experiments in skinned muscle cells.J. Physiol. 75 463–505.
FINLAYSON, B., LYMN, R. W. & TAYLOR, E. W. (1969) Studies on the kinetics of formation and dissociation of the actomyosin complex.Biochemistry,8 811–19.
FUCHS, F. (1974) Striated muscle.A. Rev. Physiol. 36 461–501.
FUCHS, F. (1977) Cooperative interactions between calcium binding sites on glycerinated muscle fibres: the influence of cross-bridge attachments.Biochim. biophys. Acta 462 314–22.
FUCHS, F. & BLACK, B. (1980) The effect of magnesium on the binding of calcium ions to glycerinated rabbit psoas muscle fibres.Biochim. biophys. Acta 622 52–62.
FUCHS, F. & BRIGGS, F. N. (1968) The site of calcium binding in relation to the activation of myofibrillar contraction.J. gen. Physiol. 51 665–76.
FUCHS, F. & FOX, C. (1982) Parallel measurements of bound calcium and force in glycerinated rabbit psoas muscle fibres.Biochim. biophys. Acta 679 110–15.
GODT, R. E. & LINDLEY, B. D. (1982) Influence of temperature upon contractile activation and isometric force production in mechanically skinned muscle fibers of the frog.J. gen. Physiol. 80 279–97.
GOLDMAN, Y. E., DANTZIG, J. A., HIBBERD, M. G. & TRENTHAM, D. R. (1983) Effects of ADP and vanadate on tension trnasients initiated by photolysis of caged-ATP.Biophys. J. 41, 147a.
GOODNO, C. C. (1979) Inhibition of myosin ATPase by vanadate ion.Proc. natn. Acad. Sci. U.S.A. 76 2620–4.
HIBBERD, M. G. & JEWELL, B. R. (1982) Calcium- and length-dependent force production in rat ventricular muscle.J. Physicol. 329 527–40.
HOLROYDE, M. J., HOWE, E. & SOLARO, R. J. (1979) Modification of calcium requirements for activation of cardiac myofibrillar ATPase by cyclic AMP dependent phosphorylation.Biochim. biophys. Acta. 586 63–9.
HOUSMANS, P. R., LEE, N. K. M. & BLINKS, J. R. (1983) Active shortening retards the decline of the intracellular calcium transient in mammalian heart muscle.Science 221 159–61.
JOHNSON, J. D., CHARLTON, S. C. & POTTER, J. D. (1979) A fluorescence stopped flow analysis of Ca2+ exchange with troponin-C.J. biol. Chem. 254 3497–502.
KAUFMANN, R. L., BAYER, R. M. & HARNASCH, C. (1972) Autoregulation of contractility in the myocardial cell: displacement as a controlling parameter.Pflügers Arch. 332 96–116.
KAWAMURA, T. & TAWADA, K. (1982) Dissociation of actomyosin by vanadate plus ADP and decomposition of the myosin-ADP-vanadate complex by actin.J. Biochem. 91 1293–8.
LYMN, R. W. & HUXLEY, H. E. (1973) X-ray diagrams from skeletal muscle in the presence of ATP analogs.Cold Spring Harb. Symp. quant. Biol. 37 449–53.
MAGID, A. & GOODNO, C. C. (1982) Inhibition of cross-bridge force by vanadate ion.Biophys. J. 37, 107a.
MOPE, L., McCLELLAN. G. B. & WINEGRAD, S. (1980) Calcium sensitivity of the contractile system and phosphorylation of troponin in hyperpermeable cardiac cells.J. gen Physiol. 75 271–82.
MOSS, R. L., SWINFORD, A. E. & GREASER, M. L. (1983) Alterations in the Ca2+ sensitivity of tension development by single skeletal muscle fibres at stretched lengths.Biophys. J. 43 115–19.
POTTER, J. D. & GERGELY, J. (1975) The calcium and magnesium binding sites on troponin and their role in the regulation of myofibrillar ATPase.J. biol. Chem. 250 4628–33.
POTTER, J. D. & JOHNSON, J. D. (1982) Troponin. InCalcium and Cell Function II (edited by CHEUNG, W. Y.), pp. 145–73. New York: Academic Press.
RIDGWAY, E. B. & GORDON, A. M. (1984) Muscle calcium transient: effect of post-stimulus length changes in single fibers.J. gen. Physiol. 83 75–103.
RIDGWAY, E. B., GORDON, A. M. & MARTYN, D. A. (1983) Hysteresis in the force-calcium relation in muscle.Science 219 1075–7.
SOLARO, R. J., HOLROYDE, M. J., HERZIG, J. W. & PETERSON, J. (1980) Cardiac relaxation and myofibrillar interaction with phosphate and vanadate.Europ. Heart J. suppl. A, 21–7.
STEPHENSON, D. G. & WILLIAMS, D. A. (1981) Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures.J. Physiol. 317 218–302.
STEPHENSON, D. G. & WILLIAMS, D. A. (1982) Effects of sarcomere length on the force-pCa relations in fast- and slow-twitch skinned muscle fibres from the rat.J. Physiol. 333 637–53.
TAYLOR, E. W. (1979) Mechanism of actomyosin ATPase and the problem of muscle contraction.CRC Crit. Rev. Biochem. 6 103–64.
TOYO-OKA, T. (1981) Calcium ion insensitive contraction of glycerinated porcine cardiac muscle fibres by Mg-inosine triphosphate.Circ. Res. 49 1350–5.
WEBER, A. (1969) Parallel response of myofibrillar contraction and relaxation to four different nucleoside triphosphates.J. gen. Physiol. 53 781–91.
WEBER, A. & MURRAY, J. M. (1973) Molecular control mechanisms in muscle contraction.Physiol. Rev. 53 612–73.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fuchs, F. The binding of calcium to detergent-extracted rabbit psoas muscle fibres during relaxation and force generation. J Muscle Res Cell Motil 6, 477–486 (1985). https://doi.org/10.1007/BF00712584
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00712584