Summary
-
1.
The effect of benzodiazepine pretreatment on the stress-induced decrease in MAO activity in rat tissues using footshock as stress model was investigated.
-
2.
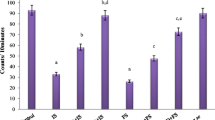
Animals were injected with vehicle, Lorazepam (1.25 mg/kg), or Clonazepam (0.5 mg/kg) 2 hr before or with PK 11195 (0.45 mg/kg) 2.5 hr before being subjected to one session of 10 inescapable footshocks or to a sham session. At the end of the session animals were sacrificed and MAO A and B activities in hearts and brains were determined.
-
3.
Pretreatment of the animals with both Lorazepam and Clonazepam abolished the decrease induced by footshock in MAO A activity in brain. Pretreatment with Lorazepam but not with Clonazepam abolished the stressinduced decrease in MAO A in the heart. Pretreatment with PK 11195 before Lorazepam reversed its effects in the heart but not in the brain. Neither footshock nor any of the drugs used had any effect on heart and brain MAO B.
-
4.
Our results suggest that in the heart but not in the brain, peripheral benzodiazepine receptors play a role in the regulation of MAO A activity under stress conditions.
Similar content being viewed by others
References
Follet, B. K. (1969). Diurnal rythms of monoamine oxidase activity in the quail hypothalamus during photoperiodic stimulation.Comp. Biochem. Physiol. 29591–600.
Urry, R. L., and Ellis, L. C. (1975). Monoamine oxidase activity in the hypothalamus and pituitary alterations after pinealectomy: Changes in photoperiod or additions of melatonin in vitro.Experientia 31891–892.
Chevillard, C., Barden, N., and Saavedra, J. M. (1981). 24 hr-rhythm in monoamine oxidase activity in specific areas of the rat brain stem.Brain Res. 223205–209.
Armando, I., Levin, G., and Barontini, M. (1988). Stress increases endogenous benzodiazepine receptor ligand-monoamine oxidase inhibitory activity (tribulin) in rat tissues.J. Neural Transm. 7129–37.
Lemoine, A. P., Armando, I., Brun, J. C., Segura, E. T., and Barontini, M. (1990). Footshock affects heart and brain MAO and MAO inhibitory activity and open field behavior in rats.Pharmacol. Biochem. Behav. 3685–88.
Boucher, T., Strolin Benedetti, M., and Tamin, C. (1987). Amine oxidase activities of interscapular brown adipose tissue of cold-exposed rats.Pharmacol. Toxicol. 607.
Maura, G., and Vaccari, A. (1975). Relationships between age and submission to environmental stress and monoamine oxidase activity in rats.Experientia 31191–193.
Clow, A., Glover, V., Oxenkrug, G. F., and Sandler, M. (1989). Stress reduces in vivo inhibition of monoamine oxidase by phenelzine in rat brain.Neurosci. Lett. 107325–330.
Becker, R. E., Giambalvo, C., Fox, R. A., and Macho, N. (1983). Endogenous inhibitors of monoamine oxidase present in human cerebrospinal fluid.Science 221476–478.
Isaac, L., Schoenbeck, R., Bacher, J., Skolnick, P., and Paul, S. M. (1986). Electroconvulsive shock increases monoamine oxidase inhibitory activity in brain and cerebrospinal fluid.Neurosci. Lett. 66257–262.
Glover, V., Reveley, M. A., and Sandler, M. (1980). A monoamine oxidase inhibitor in human urine.Biochem. Pharmacol. 29467–470.
Sandler, M. (1982). The emergence of tribulin.Trends Pharmacol. Sci. 3471–472.
Glover, V., Bhattacharya, S. K., Sandler, M., and File, S. (1981). Benzodiazepines reduce stress-augmented increase in rat urine monoamine oxidase inhibitor.Nature 292347–349.
Armando, I., Barontini, M., Levin, G., Simsolo, R., Glover, V., and Sandler, M. (1984). Exercise increases endogenous urinary monoamine oxidase-benzodiazepine receptor ligand inhibitory activity in children.J. Auton. Nerv. Syst. 1195–100.
Clow, A., Glover, V., Sandler, M., and Tiller, J. (1988). Increased urinary tribulin output in generalized anxiety disorder.Psychopharmacology 95378–380.
Armando, I., Glover, V., and Sandler, M. (1986). Distribution of endogenous benzodiazepine receptor-monoamine oxidase inhibitory activity (tribulin) in tissues.Life Sci. 382063–2067.
Sharman, D. F., Stephens, D. B., Cohen, G., and Holzbauer, M. (1987). Variations in the monoamine oxidase inhibitory activity (“tribulin?”) in pig's urine.J. Neural Transm. 69229–242.
Armando, I., Lemoine, A. P., Ferrini, M., Segura, E. T., and Barontini, M. (1989). Repeated (isolation) stress increases tribulin-like material in the rat.Cell. Mol. Neurobiol. 9115–122.
Bhattacharya, S. K., Glover, V., McIntyre, I., Oxenkrug, G., and Sandler, M. (1988). Stress causes an increase in endogenous monoamine oxidase inhibitor (tribulin) in rat brain.Neurosci. Lett. 92218–221.
Medvedev, A. E., Gorkin, V. Z., Fedotova, I. B., Semiokhina, A. F., Glover, V., and Sandler, M. (1992). Increase of brain endogenous monoamine oxidase inhibitory activity (tribulin) in experimental audiogenic seizures in rats: Evidence for a monoamine oxidase A inhibiting component of tribulin.Biochem. Pharmacol. 441209–1210.
Dionne, R. A., Goldstein, D. S., and Wirdzek, P. R. (1984). Effects of diazepam premedication and epinephrine-containing local anesthetic on cardiovascular and plasma catecholamines responses to oral surgery.Anesth. Analg. 63640–646.
LeFur, G., Guilloux, N., Mitrani, J., and Uzan, A. (1979). Relationship between plasma corticosteroids and benzodiazepines in stress.J. Pharmacol. Exp. Ther. 211305–308.
Shek, P. N., and Sabiston, B. H. (1983). Neuroendocrine regulation of immune processes: Changes in circulating corticosterone levels induce by the primary antibody response in mice.Int. J. Immunopharmacol. 523–33.
Camoratto, A. M., and Grandison, L. (1983). Inhibition of cold-induced TSH release by benzodiazepines.Brain Res. 265339–343.
Grandison, L. (1982). Supression of prolactin secretion by benzodiazepines in vivo.Neuroendocrinology 34369–373.
Squires, R., and Braestrup, C. (1977). Benzodiazepine receptors in the rat brain.Nature 266732–734.
Mohler, H., and Okada, T. (1977). Demonstration of benzodiazepine receptors in the central nervous system.Science 198849–852.
Braestrup, C., and Squires, R. F. (1977). Specific benzodiazepine receptors in rat brain characterized by high affinity3H-diazepam binding.Proc. Natl. Acad. Sci. USA 743804–3809.
Costa, E., and Guidotti, A. (1979). Molecular mechanisms in the receptor action of benzodiazepines.Annu. Rev. Pharmacol. Toxicol. 19531–545.
Haefely, W., Pieri, L., Polc, P., and Schaffner, R. (1981). General pharmacology and neuropharmacology of benzodiazepine derivatives. InHandbook of Experimental Pharmacology, Psychotropic Agents, Part 2, (F. Hoffmeisster and G. Stille, Eds.), Vol. 55, pp. 13-262.
Schofield, P. R., Darlison, M. G., Fujiata, N., Burt, D. R., Stephenson, F. A., Rodriguez, H., Rhee, L. M., Ramachadran, J., Reale, V., Glencorse, T., Seeburg, P. H., and Barnard, E. A. (1987). Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor superfamily.Nature 328221–227.
Taniguchi, T., Wang, J. K. T., and Spector, S. (1982).3H-Diazepam binding sites on rat heart and kidney.Biochem. Pharmacol. 31589–590.
Benavidez, J., Menager, J., Burgevin, M., Ferris, O., Uzan, A., Gueremy, C., Renault, C., and LeFur, G. (1985). Characterization of solubilized peripheral type benzodiazepine binding sites from adrenals by using3H PK 11195, an isoquinoline carboxamide derivative.Biochem. Pharmacol. 34167–178.
Taniguchi, T., Wang, J. K. T., and Spector, S. (1980). Properties of3H-diazepam binding to rat peritoneal mast cells.Life Sci. 27171–178.
Braestrup, C., Albechsten, R., and Squires, R. F. (1977). High densities of benzodiazepine receptors in human cortical areas.Nature 269702–704.
Marangos, P. J., Patel, J., Boulenger, J. P., and Clark-Rosenberg, R. (1982). Characterization of peripheral type benzodiazepine binding sites in brain using Ro 5-4864.Mol. Pharmacol. 2226–32.
Paul, S., Kemper, E., and Skolnick, P. (1981). In situ molecular weight determination of brain and peripheral benzodiazepine binding sites.Eur. J. Pharmacol. 76465–471.
Schoemaker, H., Bliss, M., Yamamura, S. H., and Yamamura, H. I. (1982). InBrain Neurotransmitters and Hormones (R. Collu, J. Ducharme, A. Barbeau, and G. Joulis, Raven Press, New York. Eds.), pp. 115–129.
Anholt, R. R. H., Pedersen, P. L., De Souza, E. B., and Snyder, S. H. (1987). The peripheral type benzodiazepine receptor: localization to mitochondrial outer membrane.J. Biol. Chem. 261576–583.
Mestre, M. T., Carriot, C., Belin, A., Uzan, C., Renault, M. C., Dubroeucq, C., Gueremy, C., Doble, A., and Le Fur, G. (1985). Electrophysiological and pharmacological evidence that peripheral type benzodiazepine receptors are coupled to calcium channels in the heart.Life Sci. 36391–400.
Besman, M. J., Yanagibashi, K., Lee, T. D., Kawamura, M., Hall, P. F., and Shively, J. E. (1989). Identification of des-(Gly-Ile)-endozepine as an effector of corticoptropin-dependent adrenal steroidogenesis: Stimulation of cholesterol delivery is mediated by the peripheral benzodiazepine receptor.Proc. Natl. Acad. Sci. USA 864897–4901.
Papadopoulos, V., Guarneri, P., Kreuger, K. E., Guidotti, A., and Costa, E. (1992). Pregnenolone biosynthesis in C6-2B glioma cell mitochondria: Regulation by mitochondrial diazepam binding inhibitor receptor.Proc. Natl. Acad. Sci. USA 895113–5117.
Zavala, F., Haumont, J., and Lenfant, M. (1985). Interaction of benzodiazepines with mouse macrophages.Eur. J. Pharmacol. 106561–566.
Wang, J. K. T., Morgan, J. I., and Spector, S. (1984). Benzodiazepines that binds at peripheral sites inhibit cell proliferation.Proc. Natl. Acad. Sci. USA 813770–3772.
Barbaccia, M. L., Costa, E., and Guidotti, A. (1988). Endogenous ligands for high-affinity recognition sites of psychotropic drugs.Annu. Rev. Pharmacol. Toxicol. 28451–476.
Picotti, G. B., Corli, O., Galva, M. B., Bondiolotti, G. P., and Carruba, M. O. (1982). Effects of oral chlordemethyldiazepam on plasma adrenaline and noradrenaline and cardiovascular reactivity in pre-operative patients.Eur. J. Clin. Pharmacol. 23383–388.
Elsworth, J. D., Dewar, D., Glover, V., Goodwin, B. L., Clow, A., and Sandler, M. (1986). Purification and characterization of tribulin and endogenous inhibitor of monoamine oxidase and of benzodiazepine receptor binding.J. Neural Transm. 6745–56.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Armando, I., Lemoine, A.P., Segura, E.T. et al. The stress-induced reduction in monoamine oxidase (MAO) A activity is reversed by benzodiazepines: Role of peripheral benzodiazepine receptors. Cell Mol Neurobiol 13, 593–600 (1993). https://doi.org/10.1007/BF00711559
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00711559