Summary
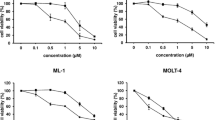
We examined the ability of lonidamine, which has been described as an inhibitor of cellular respiration and glycolysis, to enhance the cytotoxicity of alkylating agents to MCF-7 human breast-carcinoma cells. Lonidamine was increasingly cytotoxic to MCF-7 cells with increasing time of exposure. With a 12-h exposure, the IC50 for lonidamine was about 365 μM, and with a 24-h exposure it was about 170 μM. A drug concentration of 250 μM was chosen for use in the drug combination studies. Lonidamine appeared to have a dose-modifying effect on cisplatin (CDDP), producing increasingly supraadditive cell kill with increasing CDDP concentration. When simultaneously incubated with lonidamine for 1 h, 500 μM CDDP yielded a cell kill that was 2 log greater than additive cytotoxicity. Extending the exposure to lonidamine for 12 h after CDDP treatment led to a small, additional aliquot of cell kill of about 2.5-fold over the CDDP concentration range. Lonidamine also appeared to have a dose-modifying effect on melphalan cytotoxicity in the melphalan concentration range of 100–500 μM. Between concentrations of 10 and 100 μM melphalan, the drug combination survival after 1 h exposure fell within the envelope of additivity for the two agents. However, maintaining the presence of lonidamine for an additional 12 h increased the effect such that the combination was supraadditive over the entire concentration range of melphalan. Simultaneous exposure to 4-hydroperoxycyclophosphamide (4-HC) and lonidamine for 1 h resulted in greater than additive cell kill, and extending the lonidamine exposure period such that lonidamine was present during and 12 h after 4-HC treatment further increased this effect. Lonidamine had a moderate effect on the cytotoxicity of carmustine (BCNU) with a 1 h simultaneous exposure; however, this treatment combination reached greater than additive cytotoxicity only at the highest concentration of BCNU tested. Extending the lonidamine exposure time for an additional 12 h resulted in supraadditive cell kill over the BCNU concentration range. Therefore, when lonidamine was present during exposure to the alkylating agent and its presence was then extended for an additional 12 h, a synergistic cell kill was produced with all four alkylating agents tested.
Similar content being viewed by others
References
Band PR, Deschamps M, Besner JG, Leclaire R, Gervais P, DeSanctis A (1984) Phase II study of lonidamine in cancer patients Oncology 41 [Suppl 1]:66
Berenbaum MC (1977) Synergy, additivism and antagonism in immunosuppression. Clin Exp Immunol 28:1
Cavaliere R, DiFilippo F, Varanese A, Carlini S, Calabro A, Aloe L, Piarulli L (1984) Lonidamine and hyperthermia: clinical experience in melanoma; preliminary results. Oncology 41 [Suppl]:116
Deen DF, Williams MW (1979) Isobologram analysis of X-ray BCNU interactions in vitro. Radiat Res 79:483
DeMartino C, Battelli T, Paggi MG, Nista A, Marcante ML, D'Atri S, Malorni W, Gallo M, Floridi A (1984) Effects of lonidamine on murine and human tumor cells in vitro. Oncology 41 [Suppl 1]:15
DeMartino C, Malorni W, Accinni L, Rosati F, Nista A, Formisano G, Silvestrini B, Arancia G (1987) Cell membrane changes induced by lonidamine in human erythrocytes and T lymphocytes, and Ehrlich ascites tumor cells. Exp Mol Pathol 46:15
Dewey WC, Stone LE, Miller HH, Giblak RE (1971) Radiosensitization with 5-bromodeoxyuridine of Chinese hamster cells X-irradiated during different phases of the cell cycle. Radiat Res 47:672
Evans WK, Shepherd Fa, Mullix B (1984) Phase II evaluation of lonidamine in patients with advanced malignancy. Oncology 41 [Suppl 1]:69
Floridi A, Lehninger AL (1983) Action of the antitumor and antispermatogenic agent lonidamine on electron transport in Ehrlich ascites tumor mitochondria. Arch Biochem Biophys 226:73
Floridi A, Paggi MG, D'Atri S, DeMartino C, Marcante ML, Silvestrini B, Caputo A (1981) Effect of lonidamine on the energy metabolism of Ehrlich ascites tumor cells. Cancer Res 41:4661
Floridi A, Paggi MG, Marcante ML, Silvestrini B, Caputo A, DeMartino C (1981) Lonidamine, a selective inhibitor of aerobic glycolysis of murine tumor cells. J Natl Cancer Inst 66:497
Floridi A, Bagnato A, Bianchi C, Paggi MG, Nista A, Silvestrini B, Caputo A (1986) Kinetics of inhibition of mitochondrial respiration by antineoplastic agent lonidamine. J Exp Clin Cancer Res 5:273
Hahn GM, van Kersen I, Silvestrini B (1984) Inhibition of the recovery from potentially lethal damage by lonidamine. Br J Cancer 50:657
Kim JH, Alfieri A, Kim SH, Young CW, Silvestrini B (1984) Radiosensitization of Meth-A fibrosarcoma in mice by lonidamine. Oncology 41 [Suppl 1]:36
Kim JH, Kim SH, Alfieri A, Young CW, Silvestrini B (1984) Lonidamine: a hyperthermic sensitizer of HeLa cells in culture and of the Meth-A tumor in vivo. Oncology 41 [Suppl 1]: 30
Kim JH, Alfieri AA, Kim SH, Young CW (1986) Potentiation of radiation effects on two murine tumors by lonidamine. Cancer Res 46:1120
Magno L, Terraneo F, Ciottoli GB (1984) Lonidamine and radiotherapy in head and neck cancers, a pilot study. Oncology 41 [Suppl 1]:113
Murray N, Shah A, Band P (1987) Phase II study of lonidamine in patients with small cell carcinoma of the lung. Cancer Treat Rep 71:1283
Pacilio G, Carteni G, Biglietto M, DeCesare M (1984) Lonidamine alone and in combination with other chemotherapeutic agents in the treatment of cancer patients. Oncology 41 [Suppl 1]:108
Steel GG, Peckham MJ (1979) Exploitable mechanisms in combined radiotherapy-chemotherapy: the concept of additivity. Int J Radiat Oncol Biol Phys 5:85
Teicher BA, Holden SA, Cucchi CA, Cathcart KNS, Korbut TT, Flatow JL, Frei E III (1988) Combination ofN,N 1,N 11-triethylenethiophosphoramide and cyclophosphamide in vitro and in vivo. Cancer Res 48:94
Weinerman BH, Eisenhauer EA, Besner JG, Coppin CM, Stewart D, Band PR (1986) Phase II study of lonidamine in patients with metastatic renal cell carcinoma: a National Cancer Institute of Canada Clinical Trials Group study. Cancer Treat Rep 70:751
Zupi G, Greco C, Laudonio N, Benassi M, Silverstrini B, Caputo A (1986) In vitro and in vivo potentiation by lonidamine of the antitumor effect of Adriamycin. Anticancer Res 6:1245
Author information
Authors and Affiliations
Additional information
This work was supported by a grant from DeSanctis Consultants, Montreal, Canada and National Cancer Institute Grant IPOI-CA38493
Rights and permissions
About this article
Cite this article
Rosbe, K.W., Brann, T.W., Holden, S.A. et al. Effect of lonidamine on the cytotoxicity of four alkylating agents in vitro. Cancer Chemother. Pharmacol. 25, 32–36 (1989). https://doi.org/10.1007/BF00694335
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00694335