Summary
Our combined histochemical/electrophysiological examination of single, identified muscle fibers demonstrates surprising functional heterogeneity in the claw levator muscle (CL) of spider legs:
-
1.
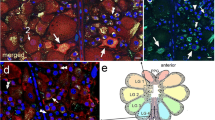
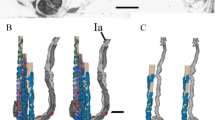
The muscle raises and retracts the claws and is also involved in tarsal depression (Fig. 1a). InCupiennius salei it is organized in 2 bilaterally asymmetrical bundles: a small ‘K-bundle’ with 37 short fibers and a relatively massive ‘L-bundle’ with 43 long fibers (Table 1).
-
2.
Histochemical staining for relative succinic dehydrogenase activity, for relative myofibrillar (actomyosin) ATPase activity (including acid preincubation), and for glycogen content in muscle cells reveals 4 distinct fiber populations A, B, C, and D, each occurring in clusters (Figs. 2, 3). A-type fibers comprise the bulk of the claw levator and occur only in the L-bundle. B-fibers appear in both muscle bundles, while the occurrence of type C and D is restricted to the K-bundle (Table 1).
-
3.
Two excitatory axons innervate the claw levator (Fig. 1 c): fast (FLE) and slow levator excitor (SLE). A-fibers are innervated by FLE alone; B and C-fibers receive innervation from both excitors; D-fibers receive excitatory innervation only by the SLE and are addtionally innervated by an inhibitory axon (Figs. 4, 5). Each fiber group differs with respect to the membrane time constantT and facilitation properties (Table 2).
-
4.
Although detailed force measurements and biochemical analyses of isolated muscle fibers are not available, we discuss how each of the 4 distinct fiber types is possibly involved with the different functions required for various locomotory behaviors ofCupiennius salei.
Similar content being viewed by others
Abbreviations
- CL :
-
claw levator
- CD :
-
claw depressor
- ejp :
-
excitatory junction potential
- ijp :
-
inhibitory junction potential
- SLE :
-
slow levator excitor
- FLE :
-
fast levator excitor
- SDH :
-
succinic dehydrogenase
References
Brenner HR (1972) Evidence for peripheral inhibition in an arachnid muscle. J Comp Physiol 80:227–231
Brooke MH, Kaiser KK (1970) Three ‘myosin adenosine triphosphate’ systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem 18:670–672
Close RI (1972) Dynamic properties of mammalian skeletal muscles. Physiol Rev 52:129–197
Costello WJ, Govind CK (1983) Contractile responses of single fibers in lobster claw closer muscles: correlation with structure, histochemistry, and innervation. J Exp Zool 227:381–393
Foelix RF, Choms A (1979) Fine structure of a spider joint receptor and associated synapses. Eur J Cell Biol 19:149–159
Fourtner CR, Sherman RG (1973) Chelicerate neuromuscular systems. Am Zool 13:271–289
Hart TF, Fourtner CR (1979) Histochemical analysis of physiologically and morphologically identified muscles in an insect leg. Comp Biochem Physiol 64:437–440
Hoyle G (1978) Distributions of nerve and muscle fibre types in locust jumping muscle. J Exp Biol 73:205–233
Linzen B, Gallowitz P (1975) Enzyme patterns in muscles of the lycosid spider,Cupiennius salei. J Comp Physiol 96:101–109
Loewe R, Linzen B, von Stackelberg W (1970) Die gelösten Stoffe in der Hämolymphe einer Spinne,Cupiennius salei Keyserling. Z Vergl Physiol 66:27–34
Maier L (1984) Histochemische und biochemische Charakterisierung identifizierter Einzelmuskelfasern bei der KrabbeEriphia spinifrons. Dissertation, Fakultät für Biologie, Universität Konstanz
Maier L, Rathmayer W, Pette D (1984) pH lability of myosin ATPase activity permits discrimination of different muscle fibre types in crustaceans. Histochemistry 81:75–77
Maier L, Pette D, Rathmayer W (1986) Enzyme activities in single electrophysiologically identified crab muscle fibres. J Physiol (Lond) 371:191–199
Melchers M (1967) Der Beutefang vonCupiennius salei Keyserling (Ctenidae). Z Morph Ökol Tiere 58:321–346
Meyer W (1981) Observations on the morphology and histochemistry of the foregut muscles of spiders (Arachnida, Araneida). J Morphol 170:113–131
Nolte J, Pette D (1972) Microphotometric determination of enzyme activity in single cells in cryostat sections II. Succinate dehydrogenase, lactate dehydrogenase and trisephosphate dehydrogenase activities in red, intermediate and white fibres of soleus and rectus femoris muscles of the rat. J Histochem Cytochem 20:577–582
Padykula HA, Herman E (1955) The specificity of the histochemical method for adenosine triphosphate. J Histochem Cytochem 3:170–195
Parry DA (1960) The small leg-nerve of spiders and a probable mechanorecetor. Q J Microsc Sci 101:1–8
Peter JB, Barnard RJ, Edgerton VR, Gillespie CA, Stempel KE (1972) Metabolic profiles of three types of skeletal muscle in guinea pigs and rabbits. Biochemistry 11:2627–2633
Rathmayer W (1965) Neuromuscular transmission in a spider and the effect of calcium. Comp Biochem Physiol 14:673–687
Rathmayer W (1966) Die Innervation der Beinmuskeln einer Spinne,Eurypelma hentzi Chamb. (Orthognatha, Aviculariidae). Verh Dtsch Zool Ges 1965:505–511
Rathmayer W, Erxleben C (1983) Identified muscle fibers in a crab. I. Characteristics of excitatory and inhibitory neuromuscular transmission. J Comp Physiol 152:411–420
Rathmayer W, Maier L (1987) Muscle fiber types in crabs: studies on single identified muscle fibers. Am Zool (in press)
Romein B (1968) Mikroskopische Technik. Oldenbourg, München, pp 275–276
Ruhland M (1976) Untersuchungen zur neuromuskulären Organisation eines Muskels aus Laufbeinregeneraten einer Vogelspinne (Dugesiella hentzi Ch.). Verh Dtsch Zool Ges 1976:238
Ruhland M, Rathmayer W (1978) Die Beinmuskulatur und ihre Innervation bei der VogelspinneDugesiella hentzi (Ch.) (Araneae, Aviculariidae). Zoomorphologie 89:33–46
Seyfarth E-A (1978) Lyriform slit sense organs and muscle reflexes in the spider leg. J Comp Physiol 125:45–57
Seyfarth E-A (1985) Spider proprioception: receptors, reflexes, and control of locomotion. In: Barth FG (ed) Neurobiology of arachnids. Springer, Berlin Heidelberg New York, pp 230–248
Seyfarth E-A, Eckweiler W, Hammer K (1985) Proprioceptors and sensory nerves in the legs of a spider,Cupiennius salei (Arachnida, Araneida). Zoomorphology 105:190–196
Sherman RG (1973) Unique arrangement of glial membranes between adjacent neuromuscular synapses in a spider muscle. J Cell Biol 59:234–238
Sherman RG (1985) Neural control of the heartbeat and skeletal muscle in spiders and scorpions. In: Barth FG (ed) Neurobiology of arachnids. Springer, Berlin Heidelberg New York, pp 319–336
Sherman RG, Luff AR (1971) Structural features of the tarsal claw muscles of the spiderEurypelma marxi Simon. Can J Zool 49:1549–1556
Speck J, Barth FG (1982) Vibration sensitivity of pretarsal slit sensilla in the spider leg. J Comp Physiol 148:187–194
Tse FW, Govind CK, Atwood HL (1983) Diverse fiber composition of swimming muscles in the blue crab,Callinectes sapidus. Can J Zool 61:52–59
Usherwood PNR, Grundfest H (1965) Peripheral inhibition in skeletal muscle of insects. J Neurophysiol 28:497–518
Wilson RS, Bullock J (1973) The hydraulic interaction between prosoma and opisthosoma inAmaurobius ferox (Chelicerata, Araneae). Z Morphol Tiere 74:221–230
Woodbury JW, Brady AJ (1956) Intracellular recording from moving tissues with flexibly mounted ultramicroelectrode. Science 123:100–101
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Maier, L., Root, T.M. & Seyfarth, EA. Heterogeneity of spider leg muscle: Histochemistry and electrophysiology of identified fibers in the claw levator. J Comp Physiol B 157, 285–294 (1987). https://doi.org/10.1007/BF00693355
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00693355