Summary
Ketone body concentration, 3-hydroxybutyrate oxidation rates in vivo and in vitro in the hepatopancreas and the activities of enzymes of ketone body metabolism in mitochondrial samples from different tissues were investigated in normal specimens ofBiomphalaria glabrata and under conditions of starvation and of infection withSchistosoma mansoni.
-
1.
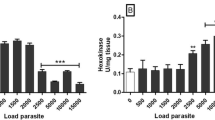
Total ketone body concentration in the hemolymph is about 700 μM in normal snails and about 80 μM in snails starved for 5 days. In the hemolymph of infected snails the concentration of total ketone bodies is at a decreased level of 400–450 μM between days 12 and 31 p.i. Significantly increased concentrations of total ketone bodies are found on days 38 and 45 p.i. with about 1300 and 950 μM, respectively.
-
2.
Oxidation rates of 3-hydroxybutyrate decrease significantly under conditions of starvation and infection compared with the normal state.
-
3.
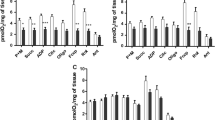
All enzymes of the HMG-CoA cycle as well as 3-oxoacid-CoA transferase are present in the hepatopancreas ofBiomphalaria glabrata. A complete HMG-CoA cycle can only be found in the hepatopancreas, other tissues lacking HMG-CoA synthase.
-
4.
In general the activities of ketone body metabolism decrease under the conditions of starvation and infection withSchistosoma mansoni. Only in the ovotestis and head-foot-region of snails infected for 31 days do the activities of acetacetyl-CoA thiolase and 3-oxoacid-CoA transferase increase as compared with the normal state.
-
5.
It is concluded that decreased ketone body concentration in the hemolymph of starved snails is due to decreased ketogenesis as a result of lacking substrate in this metabolic pathway. Increased concentrations of ketone bodies in snails infected for 38 days are mainly caused by decreased ketolytic activities in the tissues.
Similar content being viewed by others
Abbreviations
- Acac :
-
acetoacetate
- AcacCoAT :
-
acetoacetyl-CoA thiolase
- BSS :
-
balanced salt solution
- 3-HB :
-
3-hydroxybutyrate
- 3-HBDH :
-
3-hydroxybutyrate dehydrogenase
- HMG-CoA :
-
3-hydroxy-3-methylglutaryl coenzyme A
- p.i. :
-
post infectionem
- Suc-CoAT :
-
3-oxoacid-CoA transferase
References
Bailey EJ, Horne JA, Izatt MEG, Hill L (1971) Concentrations of acetacetate and 3-hydroxybutyrate in pigeon blood and desert locust hemolymph. Life Sci 10:1415–1419
Bailey EJ, Horne JA (1972) The role of ketone bodies in the metabolism of the adult desert locust. Biochem J 128:79P
Barth CA (1978) Compartmentation of ketone body synthesis in rat liver. In: Söling H-D, Seufert C-D (eds) Biochemical and clinical aspects of ketone body metabolism. Thieme, Stuttgart New York, pp 50–58
Becker W (1980) Metabolic interrelationship of parasitic trematodes and molluscs, especiallySchistosoma mansoni inBiomphalaria glabrata. Z Parasitenkd 63:101–111
Becker W, Lüth W (1977) Der Einfluß von Hunger und Infektion mitSchistosoma mansoni auf den Ketonkörpergehalt der Hämolymphe vonBiomphalaria glabrata. Z Parasitenkd 53:109–113
Beis A, Zammit VA, Newsholme EA (1980) Activities of 3-hydroxybutyrate dehydrogenase, 3-oxoacid CoA-transferase and acetoacetyl-CoA thiolase in relation to ketone-body utilisation in muscles from vertebrates and invertebrates. Eur J Biochem 104:209–215
Chernin E (1963) Observations on hearts explanted in vitro from the snailAustralorbis glabratus. J Parasitol 49:353–364
Clark JB, Nicklas WJ (1970) The metabolism of rat brain mitochondria. Preparation and characterization. J Biol Chem 245:4724–4731
Coles GC (1969) Isoenzymes of snail livers. II. Dehydrogenases. Comp Biochem Physiol 31:1–14
Dashti N, Ontko JA (1979) Rate-limiting function of 3-hydroxy-3-methylglutaryl-coenzyme A synthase in ketogenesis. Biochem Med 22:365–374
Graszynski K (1968) Enzyme des Fettsäureabbaus in den Orgagen des FlußkrebsesOrconectes limosus Rafinesque. Z Vergl Physiol 60:427–439
Graszynski K (1969) Synthese und Abbau von Ketonkörpern in den Organen des FlußkrebsesOrconectes limosus. Zool Anz Suppl 33:424–439
Graszynski K (1970a) Intrazelluläre Lokalisation von Enzymen des Flußkrebses. Enzyme der Fettsäureoxydation und des Ketonkörperstoffwechsels, Dehydrogenasen des energieliefernden Stoffwechsels und Proteasen der Mitteldarmdrüse. Z Vergl Physiol 66:107–122
Graszynski K (1970b) Ketonkörperstoffwechsel beim Flußkrebs: Stoffwechselwege, Organverteilung und physiologische Bedeutung. Z Vergl Physiol 66:439–462
Leflore WB, Fried B, Bass HS (1984) Histochemical localization of dehydrogenases in the cercaria and encysted metacercaria ofEchinostoma revolutum (Trematoda). Comp Biochem Physiol 77B:31–33
Liebsch M, Becker W, Gagelmann G (1978) An improvement of blood sampling technique forBiomphalaria glabrata using anaesthesia and long term relaxation and the role of this method in studies of regulation of hemolymph glucose. Comp Biochem Physiol 59A:169–174
Meuleman E (1972) Host-parasite interrelationships between the freshwater pulmonateBiomphalaria pfeifferi and the trematodeSchistosoma mansoni. Netherl J Zool 22:355–427
Sachs L (1970) Statistische Auswertungsmethoden. Springer, Berlin Heidelberg New York
Schacterle GL, Pollack RL (1973) A simple method for the quantitative assay of small amounts of protein in biological material. Analyt Biochem 51:654–655
Schmale H, Becker W (1975) Die Ammonium- und Harnstoffkonzentration in der Hämolymphe vonBiomphalaria glabrata unter dem Einfluß einer Infektion mitSchistosoma mansoni. Z Parasitenkd 45:237–241
Schmidt E (1970) Glutamat-Dehydrogenase. UV-Test. In: Bergmeyer HU (Hrsg) Methoden der enzymatischen Analyse, 2. Aufl. Verlag Chemie, Weinheim/Bergstraße, S 607–613
Sollock RL, Vorhaben JE, Campbell JW (1979) Transaminase reactions and glutamate dehydrogenase in gastropod hepatopancreas. J Comp Physiol 129:129–135
Stanislawski E, Becker W (1979) Influences of semi-synthetic diets, starvation and infection withSchistosoma mansoni (Trematoda) on the metabolism ofBiomphalaria glabrata (Gastropoda). Comp Biochem Physiol 63A:527–533
Stanislawski E, Becker W, Müller G (1979) Alterations of the free amino acid content in the hemolymph ofBiomphalaria glabrata (Pulmonata) in starvation and after infection withSchistosoma mansoni (Trematoda). Comp Biochem Physiol 63B:477–482
Stern JR (1956) Optical properties of acetoacetyl-S-coenzyme A and its metal chelates. J Biol Chem 221:33–44
Sugden PH, Newsholme EA (1973) Activities of hexokinase, phosphofructokinase, 3-oxo acid coenzyme A-transferase and acetoacetyl-coenzyme A thiolase in nervous tissue from vertebrates and invertebrates. Biochem J 134:97–101
Tenge A (1980) Strukturänderungen der Mitteidarmdrüse vonBiomphalaria glabrata Say, unter verschiedenen physiologischen Zuständen sowie deren Beziehungen zur Konzentration einiger Hämolymph-Metaboliten. Dissertation, Fachbereich Biologie der Universität Hamburg
Trede G, Becker W (1982) Effects of starvation and infection withSchistosoma mansoni on the release rate of free amino acids (FAA) byBiomphalaria glabrata. Comp Biochem Physiol 73B:405–409
Williamson DH, Mellanby J, Krebs HA (1962) Enzymatic determination of D-(−)-β-hydroxybutyric acid and acetoacetic acid in blood. Biochem J 82:90–96
Williamson DH, Bates MW, Krebs HA (1968) Activity and intracellular distribution of enzymes of ketone body metabolism in rat liver. Biochem J 108:353–361
Wise JB, Lehninger A (1964) In: Hoppe-Seyler, Thierfelder (Hrsg) Handbuch der physiologisch- und pathologischchemischen Analyse, Bd IV A. Springer, Berlin Göttingen Heidelberg. Cited after Graszynski K (1970b)
Author information
Authors and Affiliations
Additional information
This work was supported by the Deutsche Forschungsgemeinschaft
Rights and permissions
About this article
Cite this article
Meyer, R., Becker, W. & Klimkewitz, M. Investigations on the ketone body metabolism inBiomphalaria glabrata: Influence of starvation and of infection withSchistosoma mansoni . J Comp Physiol B 156, 563–571 (1986). https://doi.org/10.1007/BF00691043
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00691043