Abstract
The thermophilic autotrophMethanobacterium thermoautotrophicum assimilates CO2 via a novel pathway rather than via the Calvin cycle. The central intermediate of this pathway is acetyl CoA which is reductively carboxylated to pyruvate. Cell extracts of the organism contained phosphoenolpyruvate synthetase with a specific activity of 100 nmol min-1 mg-1 protein (65°C). Pyruvate kinase and pyruvate, phosphate dikinase were not detected. Phosphoenolpyruvate synthetase was partially purified (50-fold) and the following reaction stoichiometry was established:
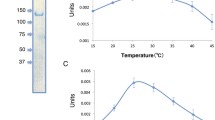
The enzyme activity was depedent on free Mg2+ ions, NH +4 or K+ ions, and SH-groups. Mn2+, but not Ca2+, could partially substitute for Mg2+; Na+ could not substitute for K+ or NH +4 . The pH-optima,V max-values and the apparentK M-values for the substrates of the enzyme in both directions were determined. Thermodynamic, kinetic and regulatory features indicate that, in vivo, the enzyme functions in the direction of phosphoenolpyruvate synthesis from pyruvate. Not only is the synthesis of phosphoenolpyruvate via the PEP synthetase reaction energetically favorable; the enzyme also catalyzed this synthesis 100 times faster than the reverse reaction, the apparentK M value for pyruvate (40 μM) being low and the apparentK M value for phosphate (100 mM) being high. Furthermore, AMP, ADP, PP and α-ketoglutarate were inhibitors of PEP synthesis, indicating that the enzyme activity may be controlled in vivo. The role of phosphoenolpyruvate synthetase in autotrophic CO2 assimilation pathway ofMethanobacterium, as expected from previous labelling studies, is confirmed.
Similar content being viewed by others
Abbreviations
- PEP:
-
Phosphoenolpyruvate
- DTE:
-
dithioerythritol
- DTT:
-
dithiothreitol
- TES:
-
N-tris-(hydrymethyl)methyl-2-aminoethanesulfonic acid
- tricine:
-
N-tris-(hydroxymethyl)methylglycine
- HFPES:
-
N-2-hydroxyethylpiperazine-N-ethanesulfonic acid
- PP:
-
pyrophosphate
References
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: Reevaluation of a unique biological group. Microbiol Rev 43:260–296
Benziman M, Palgi A (1970) Characterization and properties of the pyruvate phosphorylation system ofAcetobacter xylinum. J Bacteriol 104:211–218
Bode C, Goebbel H, Strahler E (1960) Zur Eliminierung von Trübungsfehlern bei der Eiweißbestimmung mit der Biuretmethode. Z Klin Chem Klin Biochem 6:419–422
Brandis A, Thauer RK, Stetter KO (1981) Relatedness of strains ΔH and Marburg ofMethanobacterium thermoautotrophicum. Zbl Bakt Hyg, I. Abt Orig C2:311–317
Buchanan BB (1974) Orthophosphate requirement for the formation of phosphoenolpyruvate from pyruvate by enzyme preparations from photosynthetic bacteria. J Bacteriol 119:1066–1068
Buchanan BB (1979) Ferredoxin-linked carbon dioxide fixation in photosynthetic bacteria. In: M Gibbs, E Latzko (eds) Photosynthesis II (Encyclopedia of plant physiology: new series, Vol 6). Springer, Berlin Heidelberg New York, pp 416–424
Calvin M, Bassham JA (1962) The photosynthesis of carbon compounds. WA Benjamin Inc, New York
Cooper RA, Kornberg HL (1965) Net formation of phosphoenolpyruvate from pyruvate byEscherichia coli. Biochim Biophys Acta 104:618–620
Cooper RA, Kornberg HL (1969) Phosphoenolpyruvate synthetase. In: SP Colowick, NO Kaplan (eds) Methods in enzymology, Vol 13. Academic Press, New York, pp 309–314
Cooper RA, Kornberg HL (1974) Phosphoenolpyruvate synthetase and pyruvate, phosphate dikinase. In: PD Boyer (ed) The enzymes, 3rd ed, Vol 10. Academic Press, New York, pp 631–649
Chulavatnatol M, Atkinson DE (1973) Phosphoenolpyruvate synthetase fromE. coli. Effects of adenylate energy charge and modifier concentrations. J Biol Chem 248:2712–2715
Daniels L, Zeikus JG (1978) One-carbon metabolism in methanogenic bacteria: Analysis of short-term fixation products of14CO2 and14CH3OH incorporated into whole cells. J Bacteriol 136:75–84
Evans HJ, Wood HG (1968) P-enolpyruvate synthesis from pyruvate. Fed Proc 27:588
Evans MCW, Buchanan BB, Arnon DI (1966) A new ferredoxin dependent carbon reduction cycle in a photosynthetic bacterium. Proc Natl Acad Sci 55:928–934
Eyzaguirre J (1979) Studies on bacterial pyruvate kinase: Properties of the enzyme fromPseudomonas aeruginosa andThermus thermophilus. Arch Biol Med Exper 12:605–610
Ferdinand W (1976) The enzyme molecule, p 217. John Wiley & Sons, New York
Frings W, Schlegel HG (1971) Synthese von Phosphoenolpyruvat aus Pyravat durch Extrakte ausHydrogenomonas eutropha. Arch Microbiol 79:220–230
Fuchs G, Stupperich E (1978) Evidence for an incomplete reductive carboxylic acid cycle inMethanobacterium thermoautotrophicum. Arch Microbiol 118:121–125
Fuchs G, Stupperich E (1980) Acetyl CoA, a central intermediate of autotrophic CO2 fixation inMethanobacterium thermoautotrophicum. Arch Microbiol 127:267–272
Fuchs G, Stupperich E, Jaenchen R (1980b) Autotrophic CO2 fixation inChlorobium limicola. Evidence against the operation of the Calvin cycle in growing cells. Arch Microbiol 128:56–63
Fuchs G, Stupperich E (1982) Autotrophic CO2 fixation pathway inMethanobacterium thermoautotrophicum. Zbl Bakt Hyg I. Abt Orig C 3:277–288
Fuchs G, Stupperich E, Thauer RK (1978) Acetate assimilation and the synthesis of alanine, aspartate and glutamate inMethanobacterium thermoautotrophicum. Arch Microbiol 117:61–66
Fuchs G, Stupperich E, Eden G (1980a) Autotrophic CO2 fixation inChlorobium limicola. Evidence for the operation of a reductive tricarboxylic acid cycle in growing cells. Arch Microbiol 128:64–71
Hatch MD, Slack CR (1968) A new enzyme for the interconversion of pyruvate and phosphopyruvate and its role in the C4 dicarboxylic acid pathway of photosynthesis. Biochem J 106:141–146
Ivanovsky RN, Sintsov NV, Kondratieva EN (1980) ATP-linked citrate lyase activity in the green sulfur bacteriumChlorobium limicola formathiosulfatophilum. Arch Microbiol 128:239–241
Leloir LF, Cardini CE (1957) Characterization of phosphorus compounds by acid lability. In: SP Colowick, NO Kaplan (eds) Methods in enzymology, Vol 3. Academic Press, New York, pp 840–850
Pfennig N, Trüper HG (1974) The phototrophic bacteria. In: RE Buchanan, NE Gibbons (eds) Bergey's manual of determinative bacteriology, 8 ed. Williams and Wilkins Co, Baltimore, pp 24–64
Quayle JR, Ferenci T (1978) Evolutionary aspects of autotrophy. Microbiol Rev 42:251–273
Reeves RE (1968) A new enzyme with the glycolytic function of pyruvate kinase. J Biol Chem 243:3202–3204
Schönheit P, Moll J, Thauer RK (1979) Nickel, cobalt, and molybdenum requirement for growth ofMethanobacterium thermoautotrophicum. Arch Microbiol 123:105–107
Schönheit P, Moll J, Thauer RK (1980) Growth parameters (K s , μmax,Y s) ofMethanobacterium thermoautotrophicum. Arch Microbiol 127: 59–65
Sols A (1981) Multimodulation of enzyme activity. Current topics in cellular regulation 19:77–101
Stupperich E, Fuchs G (1981) Products of CO2 fixation and14C labelling pattern of alanine inMethanobacterium thermoautotrophicum pulse-labelled with14CO2. Arch Microbiol 130:294–300
Taylor GT, Kelly DP, Pirt SJ (1976) Intermediary metabolism in methanogenic bacteria. In: HG Schlegel, G Gottschalk, N Pfennig (eds) Proceedings of the symposium “Microbial production and utilization of gases (H2, CH4, CO)”. Akademie der Wissenschaften zu Göttingen, E. Goltze Verlag, Göttingen, pp 173–180
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic bacteria. Bacteriol Rev 41:100–180
Zeikus JG, Fuchs G, Kenealy W, Thauer RK (1977) Oxidoreductases involved in cell carbon synthesis ofMethanobacterium thermoautotrophicum. J Bacteriol 132:604–613
Zeikus JG, Wolfe RS (1972)Methanobacterium thermoautotrophicum sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol 109:707–713
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eyzaguirre, J., Jansen, K. & Fuchs, G. Phosphoenolpyruvate synthetase inMethanobacterium thermoautotrophicum . Arch. Microbiol. 132, 67–74 (1982). https://doi.org/10.1007/BF00690820
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00690820