Abstract
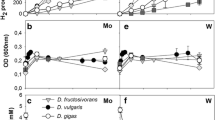
Two of nine sulfate reducing bacteria tested,Desulfobulbus propionicus andDesulfovibrio desulfuricans (strain Essex 6), were able to grow with nitrate as terminal electron acceptor, which was reduced to ammonia.
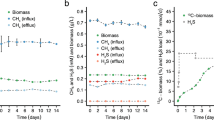
Desulfovibrio desulfuricans was grown in chemostat culture with hydrogen plus limiting concentrations of nitrate, nitrite or sulfate as sole energy source. Growth yields up to 13.1, 8.8 or 9.7 g cell dry mass were obtained per mol nitrate, nitrite or sulfate reduced, respectively. The apparent half saturation constants (K s) were below the detection limits of 200, 3 or 100 μmol/l for nitrate, nitrite of sulfate, respectively. The maximum growth rates {ie63-1} raised from 0.124 h-1 with sulfate and 0.150 h-1 with nitrate to 0.193 h-1 with nitrite as electron acceptor. Regardless of the electron acceptor in the culture medium, cell extracts exhibited absorption maxima corresponding to cytochromec and desulfoviridin. Nitrate reductase was found to be inducible by nitrate or nitrite, whereas nitrite reductase was synthesized constitutively. The activities of nitrate and nitrite reductases with hydrogen as electron donor were 0.2 and 0.3 μmol/min·mg protein, respectively. If limiting amounts of hydrogen were added to culture bottles with nitrate as electron acceptor, part of the nitrate was only reduced to the level of nitrite. In media containing nitrate plus sulfate or nitrite plus sulfate, sulfate reduction was suppressed.
The results demonstrate that the ammonification of nitrate or nitrite can function as sole energy conserving process in some sulfate-reducing bacteria.
Similar content being viewed by others
References
Badziong W, Thauer RK (1980) Vectorial electron transport inDesulfovibrio vulgaris (Marburg) growing on hydrogen plus sulfate as sole energy source. Arch Microbiol 125:167–174
Barton LL, LeGall J, Odom JM, Peck HD (1983) Energy coupling to nitrite respiration in the sulfate-reducing bacteriumDesulfovibiro gigas. J Bacteriol 153:867–871
Bokranz M, Katz J, Schröder I, Robertson AM, Kröger A (1983) Energy metabolism and biosynthesis ofVibrio succinogenes growing with nitrate or nitrite as terminal electron acceptor. Arch Microbiol 135:36–41
Cataldo DA, Haroon M, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6:71–80
Chance B, Williams GR (1956) The respiratory chain and oxidative phosphorylation. Adv Enzymol 17:65–134
Chaney AL, Marbach EP (1962) Modified reagents for the determination of urea and ammonia. Clin Chem 8:130–132
Cline E (1969) Spectrophotometric determination of hydrogen-sulfide in natural waters. Limnol Oceanogr 14:454–458
Cypionka H (1986) Sulfide-controlled continuous culture of sulfate reducing bacteria. J Microbiol Methods 5:1–9
Cypionka H, Pfennig N (1986) Growth yields ofDesulfotomaculum orientis with hydrogen in chemostat culture. Arch Microbiol 143:396–399
De Vries W, Niekus HGD, Boellaard M, Stouthamer AH (1980) Growth yields and energy generation byCampylobacter sputorum subspeciesbubulus during growth in continuous culture with different hydrogen acceptors. Arch Microbiol 124:221–227
De Vries W, Niekus HGD, van Berchum H, Stouthamer AH (1982) Electron transport-linked proton translocation at nitrite reduction inCampylobacter sputorum subspeciesbubulus. Arch Microbiol 131:132–139
Freier RK (1964) Wasseranalyse. de Gruyter, Berlin
Keith SM, Herbert RA (1983) Dissimilatory nitrate reduction by a strain ofDesulfovibrio desulfuricans. FEMS Microbiol Lett 18:55–59
King D, Nedwell DB (1985) The influence of nitrate concentration upon the end-products of nitrate dissimilation by bacteria in anaerobic salt marsh sediment. FEMS Microbiol Ecol 31:23–28
Koike I, Hattori A (1978) Denitrification and ammonia formation in anaerobic coastal sediments. Appl Environ Microbiol 35:278–282
Klemps R, Cypionka H, Widdel F, Pfennig N (1985) Growth with hydrogen, and further physiological characteristics ofDesulfotomaculum species. Arch Microbiol 143:203–208
Mcfarlane GT, Herbert RA (1984) Dissimilatory nitrate reduction and nitrification in estuarine sediments. J Gen Microbiol 130:2301–2308
McCready RGL, Gould WD, Cook FD (1983) Respiratory nitrate reduction byDesulfovibrio sp. Arch Microbiol 135:182–185
Miller JDA, Wakerley DS (1966) Growth of sulphate-reducing bacteria by fumarate dismutation. J Gen Microbiol 43:101–107
Miller JDA, Neumann PM, Elford L, Wakerley DS (1970) Malate dismutation byDesulfovibrio. Arch Mikrobiol 71:214–219
Mitchell GJ, Jones JG, Cole JA (1986) Distribution and regulation of nitrate and nitrite reduction byDesulfovibrio andDesulfotomaculum species. Arch Microbiol 144:35–40
Nethe-Jaenchen R, Thauer RK (1984) Growth yields and saturation constant ofDesulfovibrio vulgaris in chemostat culture. Arch Microbiol 137:236–240
Pirt SJ (1965) The maintenance energy of bacteria in growing cultures. Proc Roy Soc B 163:224–231
Pirt SJ (1975) Principles of microbe and cell cultivation. Blackwell, Oxford.
Postgate JR (1956) Cytochromec 3 and desulphoviridin; pigments of the anaerobicDesulphovibrio desulphuricans. J Gen Microbiol 14:545–572
Postgate JR (1984) The sulphate-reducing bacteria. 2nd ed. Cambridge University Press
Schmidt K, Liaaen-Jensen S, Schlegel HG (1963) Die Carotinoide der Thiorhodaceae. I. Okenon als Hauptcarotinoid vonChromatium okenii Perty. Arch Mikrobiol 46:117–126
Steenkamp DJ, Peck HD (1981) Proton translocation associated with nitrite respiration inDesulfovibrio desulfuricans. J Biol Chem 256:5450–5458
Sørensen J (1978) Capacity for denitrification and reduction of nitrate to ammonia in a coastal marine sediment. Appl Environ Microbiol 35:301–305
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Widdel F (1980) Anaerober Abbau von Fettsäuren und Benzoesäure durch neu isolierte Arten Sulfat-reduzierender Bakterien. Ph. D. Thesis, University of Göttingen, FRG.
Widdel F, Pfennig N (1982) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. II. Incomplete oxidation of propionate byDesulfobulbus propionicus gen. nov. sp. nov. Arch Microbiol 131:360–365
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Seitz, HJ., Cypionka, H. Chemolithotrophic growth ofDesulfovibrio desulfuricans with hydrogen coupled to ammonification of nitrate or nitrite. Arch. Microbiol. 146, 63–67 (1986). https://doi.org/10.1007/BF00690160
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00690160